Faculty

Harvard Medical School – Brigham and Women’s Hospital
Cambridge, MA

Mayo Clinic College of Medicine and Science
Mayo Clinic Arizona
Scottsdale, AZ

Goal
The goal of this program is to educate clinicians about new data supporting the use of microbiota-based therapies in the prevention of recurrent Clostridioides difficile infection (rCDI) and how these agents can be integrated into clinical patient care.
Intended Audience
The intended audience for this activity comprises gastroenterologists and infectious disease (ID) physicians; nurse practitioners, physician assistants, and registered nurses in the gastroenterology and ID fields; ID pharmacists; hospital/health-system pharmacy directors/chiefs and formulary directors/chiefs; and specialty pharmacists/infusion providers.
Educational Objectives
At the completion of the activity, participants will be better able to:
- Describe the pathophysiology and health burdens of rCDI, as well as severe and fulminant Clostridioides difficile infection (CDI).
- Evaluate the most recent clinical trial data on new and emerging microbiota-based therapies for the prevention of rCDI.
- Examine the practical applications of microbiota-based therapies for rCDI and their positioning within clinical practice guidelines.
- Discuss the rationale for inclusion of microbiota-based therapies in formularies.
Physician Accreditation Statement
This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Global Education Group (Global) and Applied Clinical Education (ACE). Global is accredited by the ACCME to provide continuing medical education for physicians.
Physician Credit Designation
Global designates this enduring activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Pharmacist Accreditation Statement
Global Education Group is accredited by the Accreditation Council for Pharmacy Education (ACPE) as a provider of continuing pharmacy education with Commendation.
Pharmacist Credit Designation
Global Education Group designates this continuing education activity for 1.0 contact hour (0.1 CEU) of the ACPE (Universal Activity Number 0530-9999-25-025-H01-P). This is a knowledge-based activity.
Instructions For Obtaining Credit
To receive credit, participants must participate in the activity and complete and pass the post-test with a minimum score of 70%. Additionally, participants will need to complete the evaluation. CME certificates will be sent via email to those successfully completing the activity.
Disclosures of Relevant Financial Relationships
Global adheres to the policies and guidelines, including the Standards for Integrity and Independence in Accredited CE, set forth to providers by the ACCME and all other professional organizations, as applicable, stating those activities where continuing education credits are awarded must be balanced, independent, objective, and scientifically rigorous. All persons in a position to control the content of an accredited continuing education program provided by Global are required to disclose all financial relationships with any ineligible company within the past 24 months to Global. All financial relationships reported are identified as relevant and mitigated by Global in accordance with the Standards for Integrity and Independence in Accredited CE in advance of delivery of the activity to learners. The content of this activity was vetted by Global to assure objectivity and that the activity is free of commercial bias. All relevant financial relationships have been mitigated.
The faculty have the following relevant financial relationships with ineligible companies:
- Jessica R. Allegretti, MD: Consulting fees (eg, advisory boards): AbbVie, Bristol Myers Squibb, Celltrion, Ferring, Genentech, GlaxoSmithKline, Janssen, Merck, Pfizer, Roivant, Seres Therapeutics, Shattuck Labs, TRXBio, Vedanta, Xencor; contracted research (principal investigators must provide information, even if received by the institution): Abbvie, Janssen, Merck, Pfizer, Sanofi, Takeda; speakers’ bureaus: AbbVie, Janssen
- Robert Orenstein, DO: Nothing to disclose
- Ann Crotts, PharmD, BCIDP: Nothing to disclose
The planners and managers have the following relevant financial relationships with ineligible companies:
- The planners and managers at Global have no relevant financial relationships to disclose.
- The planners and managers at ACE have no relevant financial relationships to disclose.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents not indicated by the FDA. Global and ACE do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of any organization associated with this activity. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed in this activity should not be used by clinicians without evaluation of patient conditions and possible contraindications on dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
System Requirements
PC
- 1.4 GHz Intel Pentium 4 or faster processor (or equivalent)
- Windows 10, 8.1 (32-bit/64-bit); Windows 7 (32-bit/64-bit) 512 MB of RAM (1 GB recommended)
- Microsoft Internet Explorer 11 or later, Windows Edge browser, Mozilla Firefox, and Google Chrome
- For HTML Client – Google Chrome (v70.0 and above), Mozilla Firefox (v65.0 and above), and Edge (v42.0 and above)
Mac
- 1.83 GHz Intel Core Duo or faster processor 512 MB of RAM (1 GB recommended)
- MAC OS X 10.12, 10.13, and 10.14
- Mozilla Firefox, Apple Safari, Google Chrome
- For HTML Client – Google Chrome (v70.0 and above), Apple Safari (v12.0 and above), and Mozilla Firefox (v65.0 and above)
Global Contact Information
For information about the accreditation of this program, please contact Global at (303) 395-1782 or cme@globaleducationgroup.com.
Fee Information and Refund/Cancellation Policy
There is no fee for this educational activity.
This activity is jointly provided by Global Education Group and Applied Clinical Education.

This activity is supported by an educational grant from Ferring Pharmaceuticals.
This activity is distributed by Pharmacy Practice News, Gastroenterology & Endoscopy News, Infectious Disease Special Edition, Specialty Pharmacy Continuum, and CMEZone.com.

false
*You must be logged in to see results data.
Clostridioides difficile is an anaerobic, gram-positive, spore-forming bacillus commonly found in health care settings, the environment, and food supplies.1 Although colonization by C. difficile is common and often asymptomatic, C. difficile infection (CDI) can lead to toxin-mediated intestinal injury. This infection presents a clinical spectrum that ranges from mild, self-limited diarrhea to severe conditions like fulminant colitis, toxic megacolon, sepsis, and even death.2 CDI poses a significant public health threat due to its significant morbidity, high risk for recurrence, and considerable clinical and economic burdens.
Recurrent CDI (rCDI) is particularly problematic, often stemming from the inherent limitations of standard antibiotic regimens. Although antibiotics such as vancomycin and fidaxomicin are effective in resolving acute CDI episodes, they can also disrupt the gut microbiota, impairing colonization resistance and predisposing patients to recurrent infections. This awareness has spurred the development of microbiota-targeted therapeutics aimed at re-establishing microbial homeostasis. Since 2022, two microbiota-based products have received FDA approval for the prevention of rCDI, representing a paradigm shift in treatment strategy. Additional candidates, employing diverse mechanisms of microbiome restoration, are currently undergoing clinical investigation.
Acronyms used in this activity
| ACG | American College of Gastroenterology | |
| AE | Adverse event | |
| AGA | American Gastroenterological Association | |
| CDC | Centers for Disease Control and Prevention | |
| CDI | Clostridioides difficile infection | |
| CFU | Colony-forming unit | |
| CM | Clinical modification | |
| ED | Emergency department | |
| FMT | Fecal microbiota transplantation | |
| GI | Gastrointestinal | |
| HAI | Health care-associated infection | |
| HrQoL | Health-related quality of life | |
| IBD | Inflammatory bowel disease | |
| ICD | International Classification of Diseases | |
| IDSA | Infectious Diseases Society of America | |
| mAb | Monoclonal antibody | |
| MET | Microbial ecosystem therapeutic | |
| PCR | Polymerase chain reaction | |
| QoL | Quality of life | |
| rCDI | Recurrent CDI | |
| SF-36v2 | 36-Item Short-Form Health Survey version 2 | |
| SHEA | Society for Healthcare Epidemiology of America | |
| TEAE | Treatment-emergent adverse event | |
| TRAE | Treatment-related adverse event |
In 2019, the Centers for Disease Control and Prevention (CDC) identified C. difficile as one of the 5 pathogens that pose an urgent threat to public health in the United States.3 Although C. difficile is not inherently resistant to antimicrobials, it was included in this category due to its strong association with antibiotic exposure and substantial clinical and epidemiologic effects.
Annually, more than 460,000 cases and an estimated 30,000 deaths in the United States are attributed to CDI.4,5 C. difficile is the leading cause of health care- and antibiotic-associated infective diarrhea in the country.5 A study conducted in 2015 by the CDC’s Emerging Infections Program revealed that C. difficile accounted for 15% of the health care-associated infections (HAIs) across 199 geographically diverse hospitals making it the most common pathogen responsible for HAIs nationwide.6
CDI was once mainly viewed as an HAI that significantly affected patients in health care settings and long-term care facilities. However, efforts aimed at preventing HAIs and improving antibiotic stewardship—particularly regarding fluoroquinolones—have effectively reduced the number of CDI cases linked to health care settings.4 In contrast, the incidence of community-acquired CDI cases in the United States has increased from 170,000 annually in 2011 to 226,400 in 2017 (Figure 1).4
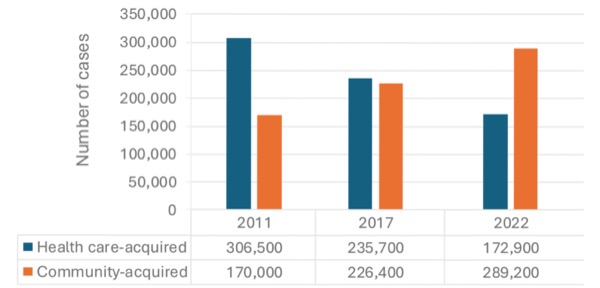 |
| Figure 1. Annual estimated US cases of Clostridioi difficile infection.4,7 |
According to CDC surveillance data, community-acquired cases of CDI outnumbered health care-associated cases in 2022, with rates of 62.1 and 54 per 100,000 individuals, respectively.4,7 Notably, only 56% of cases had a reported history of antibiotic use in the 12 weeks before infection.7 Community-acquired CDI occurs in individuals of all age groups from infants to the elderly, although those with community-acquired CDI tend to be younger than those with health care-associated CDI.8
CDI is more prevalent and often more severe in older individuals; however, the number of cases among younger patients is on the rise.3-5 According to data from a large sample of hospitalized patients with CDI (derived from ICD-9/10-CM code data), the percentage of cases in patients younger than 50 years of age increased from 12.6% in 2007 to 18.1% in 2017.9
CDI most often develops after a patient has received treatment with antibiotics. However, it can also occur in individuals with no recent history of antibiotic exposure.5,7 A significant percentage of neonates and infants are asymptomatically colonized by C. difficile, as are hospitalized adults and those residing in long-term care settings.8,10
The transmission of C. difficile primarily occurs through the fecal–oral route, either from the environment to person or from person to person. The organism can be transmitted in its vegetative form or as spores, which can persist in the environment for long periods.8,11 The persistence of C. difficile spores and the prevalence of asymptomatic colonization have presented challenges for infection control in health care settings. However, effective infection control measures and recommendations have been established to address these challenges.12
Potential sources of community-acquired CDIs include transmission from asymptomatic carriers, particularly infants and young children; occupational exposures in health care settings; exposure as a patient in outpatient health care settings; and possibly, food-borne or zoonotic sources.8,13
Recurrent CDI is common, with an estimated 75,000 to 175,000 cases occurring each year in the United States.5 Between 20% and 35% of patients who experience their first episode of CDI will have a recurrence, typically within 30 days. Furthermore, more than 60% of these patients will go on to experience additional recurrences of CDI.5
Individuals who have experienced 2 or more recurrences of CDI face more than double the risk for additional recurrences.5 This risk is also increased in certain populations, including women; older adults; those with certain comorbidities such as inflammatory bowel disease (IBD), malignancies, or renal issues; those undergoing chemotherapy treatment; and those who are immunosuppressed. Additionally, patients receiving treatments like antibiotics, corticosteroids, lipid-lowering medications, or proton-pump inhibitors are at an increased risk for CDI.5
C. difficile infections can present a range of symptoms, from mild to severe, and lead to fulminant disease.10 Mild to moderate cases typically include diarrhea and cramping abdominal pain, with most patients recovering within 3 to 5 days after antibiotic treatment. In contrast, more serious cases may lead to severe diarrhea, fever, abdominal pain and distension, nausea, vomiting, circulatory shock, and other complications.5
Severe or fulminant CDI affects an estimated 8% of individuals hospitalized with CDI.14 Severe CDI is characterized by a white blood cell count of ≥15,000 cells/mL and/or a serum creatinine level >1.5 mg/dL.15,16 Fulminant CDI, previously known as severe, complicated CDI, is characterized by severe symptoms accompanied by shock, ileus, or megacolon.15,16 It accounts for approximately 3% to 5% of CDI cases and is associated with mortality rates ranging between 30% and 40%.17 Patients with fulminant CDI often require mechanical ventilation, vasopressor support, and extended stays in the intensive care unit. Many of these patients may need surgery, such as colectomy or diverting loop ileostomy. Although these procedures can be potentially curative, they also pose significant risks, including high rates of morbidity and in-hospital mortality.17-19
Complications of CDI and rCDI can include megacolon, intestinal perforation, toxic gastroenteritis or colitis, colectomy, renal failure, sepsis megacolon, intestinal perforation, and kidney failure. Bloodstream infections may arise due to the disruption of the gastrointestinal (GI) epithelium by CDI toxins, leading to the translocation of bacteria.17,20-22
Mortality
Estimates of attributable mortality from CDI have varied between 4.5% and 16.7%, based on data published since 2000.3,5,23,24
Recurrent CDI is linked to a higher risk for complications and increased mortality.20 Research indicates that CDI-related deaths among Medicare beneficiaries aged 65 years and older are nearly 10 times more frequent within 1 year after experiencing rCDI (25.4%) compared with nonrecurrent CDI (2.7%). Furthermore, the risk for mortality in these older patients increases with the number of recurrences: It starts at 16% for 1 recurrence, climbs to 31% for 2 recurrences, and reaches 39% for 3 or more recurrences (Figure 2).21,25
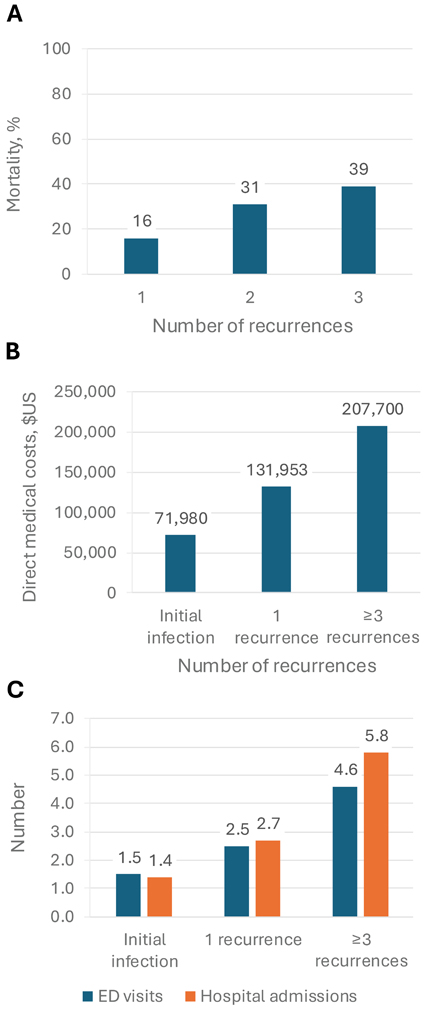 |
| Figure 2. Burdens associated with CDI and rCDI.21,25
A. Mortality in Medicare beneficiaries aged ≥65 years;
|
Social and Mental Health Burden of rCDI
Patients with CDI and rCDI often experience significant challenges across various domains, including social, mental health, and overall quality of life (QoL). In a cross-sectional, survey-based study of US adults who currently have or have had CDI, participants reported that the infections affected multiple aspects of their lives. This included daily functioning, physical health, psychological well-being, finances, work activities, and social interactions. Notably, those who experienced CDI recurrence reported significantly higher levels of interference in their physical health, psychological well-being, daily life, and work activities. Overall, 87.2% of respondents with a history of CDI expressed fear of recurrence.26
An analysis of data from the National Health and Wellness Survey, conducted across 8 countries between 2013 and 2016, revealed that individuals who had a current or past diagnosis of CDI reported lower health-related QoL (HrQoL) across all 8 domains of the 36- Item Short-Form Health Survey version 2 (SF- 36v2) compared with those who did not. After adjustments for age, sex, educational attainment, country, and scores on the Charlson Comorbidity Index, it was found that respondents with current or past CDI had lower scores on both the SF-36v2 mental and physical component summaries compared with those who had never experienced a CDI. Notably, individuals with current CDI scored the lowest across all domains.27
Economic Burden of rCDI
The direct economic cost of CDI to the US health care system is estimated to be $4.8 billion annually. Within this, the cost associated with rCDI is estimated at $2.8 billion per year. Hospitalization costs constitute the largest proportion of these expenses, followed by surgical costs.28,29
According to the CDC, based on large, nationally representative studies, the average cost of a single case of hospital-onset CDI was $12,675 in 2017 dollars. This figures represents only the costs incurred during that hospitalization.3 In addition to the costs associated with inpatient care, CDI also imposes additional financial burdens related to surgeries, emergency department (ED) visits, physician office consultations, pharmacy expenses, laboratory tests, and imaging studies.5
A systematic review and cost synthesis of 31 studies conducted in the United States and published or presented between 2012 and 2022 estimated that the direct medical costs attributable to rCDI range from $67,837 to $82,268 per patient per year.1
Focusing specifically on US adults aged 18 to 64 years, a retrospective analysis of commercial claims data revealed that patients without CDI recurrence had average all-cause direct medical costs of $71,980 in the 12 months after the initial infection. In contrast, patients with a single recurrence had mean costs of $131,953, whereas those with 3 or more recurrences had mean costs of $207,700 during the same follow-up period (Figure 2).25 The analysis highlighted the significant increase in health care resource consumption associated with rCDI. Patients with 3 or more CDI recurrences had an average of 4.6 ED visits and 5.8 hospital admissions within a 12-month follow-up period. In contrast, patients with a single recurrence had 2.5 ED visits and 2.7 admissions, whereas those without any recurrences averaged 1.5 ED visits and 1.4 admissions.25
Beyond direct health care costs, CDI and rCDI can impose substantial financial and social burdens on both patients and their caregivers. These burdens include missed work and out-of-pocket expenses.5,26,30 In a survey-based study, 47.2% of respondents with a history of CDI reported being unable to work during their active infection for an average of 118 days (median of 60 days). Additionally, 25.5% were unable to work even after clearance of the infection for a mean of 310 days (median of 62.5 days). Respondents with a history of CDI also reported mean out-of-pocket expenses of $8695 (median of $2500).26
The GI tract is a complex environment that hosts bacteria essential for human health. One of the key functions of the gut microbiota is to provide resistance against harmful organisms, such as C. difficile.31 This resistance is achieved through several mechanisms.
A diverse gut microbiota acts as an ecological barrier against pathogenic infections by competing for resources and niches, producing antimicrobial substances, and regulating the immune system (Figure 3). In addition, gut microbiota members play a crucial role in the metabolism of bile acids, which are water-soluble molecules derived from cholesterol that aid in digestion. Primary bile acids are synthesized in the liver and secreted into the small intestine, where various microbial species modify them into secondary bile acids. Whereas primary bile acids promote C. difficile spore germination, secondary bile acids inhibit spore germination and suppress the growth of vegetative C. difficile cells.10,31
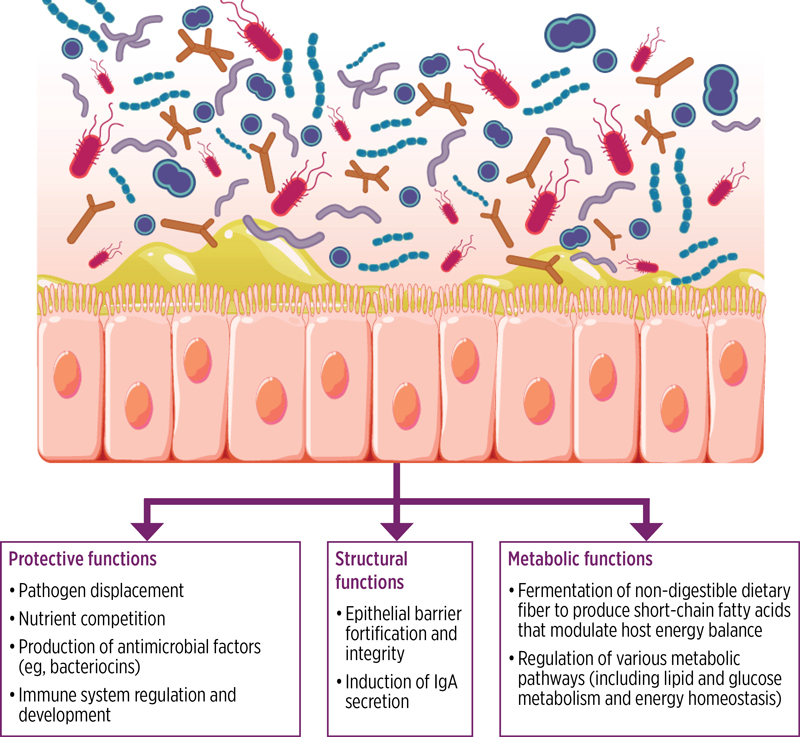 |
| Figure 3. Protective, structural, and metabolic functions of the gut microbiota to promote overall health. A healthy microbiota consists of a broad consortium of organisms that promote overall health through protective, structural, and metabolic functions.31 IgA, immunoglobulin A. Reproduced with permission. Creative Commons https://creativecommons.org/licenses/by-nc/4.0/ |
The human gut harbors more than 2000 microbial species, primarily from the bacterial phyla Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria. Although interindividual variation exists, healthy individuals typically have a gut microbiota dominated by Bacteroidetes and Firmicutes, with Proteobacteria and Actinobacteria present in smaller amounts.31
Disruption of the gut microbiota’s composition and function, a condition known as dysbiosis, can be caused by various factors, including antibiotic use (Figure 4). Dysbiosis weakens the gut’s ecological barrier, increasing the risk for CDI by enabling C. difficile spore germination and proliferation.31 Dysbiosis is characterized by shifts in the relative abundance of bacterial phyla, reduced microbial diversity, and alterations in metabolic pathways. Additionally, an increased ratio of primary to secondary bile acids, resulting from the loss of microbial taxa, may promote the germination of C. difficile spores. Other metabolic changes involving microbial-derived metabolites, such as amino acids, sialic acid, and succinate, can also contribute to increased CDI susceptibility.10,31
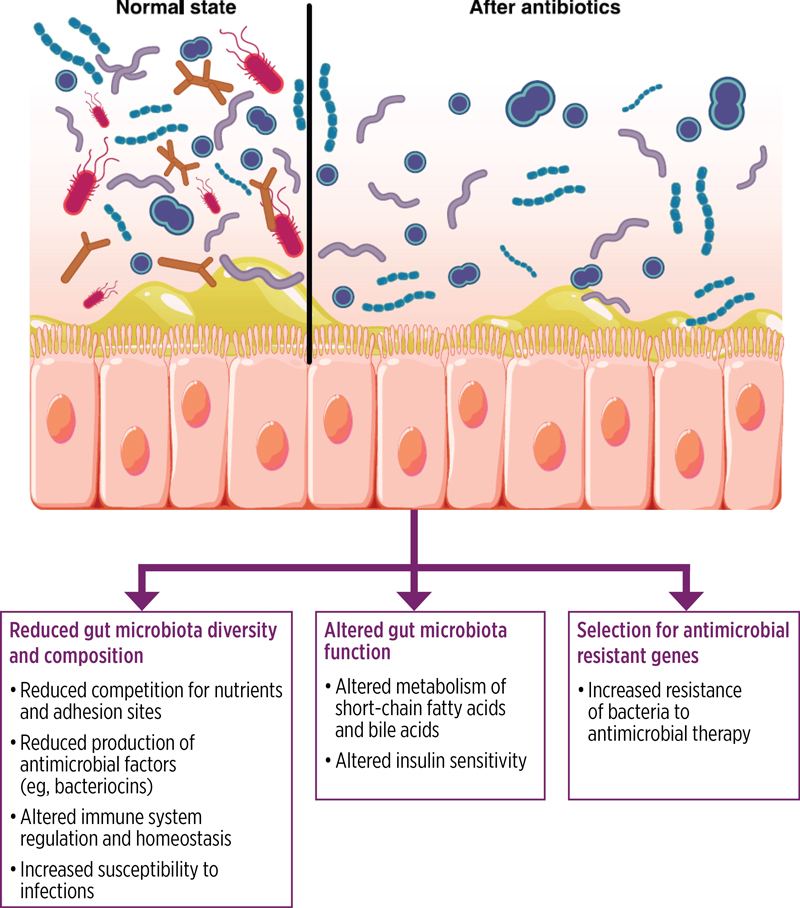 |
| Figure 4. Effect of antibiotics on gut microbiota. Antibiotics disrupt the gut microbiota; reduce diversity, composition, and function; reduce colonization resistance against potential pathogens such as Clostridioides difficile; and select for antimicrobial-resistant organisms and genes.31 |
Antibiotic use significantly affects the gut microbiota, disrupting the balance of the microbial community and potentially leading to dysbiosis, which increases the risk for CDI. The antibiotics most strongly associated with this risk include clindamycin, cephalosporins, ampicillin, and fluoroquinolones.10 However, it is important to note that nearly any antibiotic has the potential to induce CDI.32 Patients with CDI or antibiotic-associated diarrhea from other pathogens typically show a significant reduction in the abundance of Bacteroidetes and Firmicutes. This is accompanied by an overall decline in gut microbial diversity. Additionally, these patients often exhibit an increased relative abundance of Enterococcus.31
After ingestion, C. difficile spores can germinate in the GI tract when conditions are favorable to develop metabolically active and replicating vegetative forms. In the gut, these vegetative cells of toxigenic C. difficile strains produce toxins that contribute to pathogenesis (Figure 5).11,31 The pathophysiology of CDI is directly linked to the production of these toxins, which include toxin A (TcdA), toxin B (TcdB), and, in some strains, a binary toxin that can enhance virulence.11,31
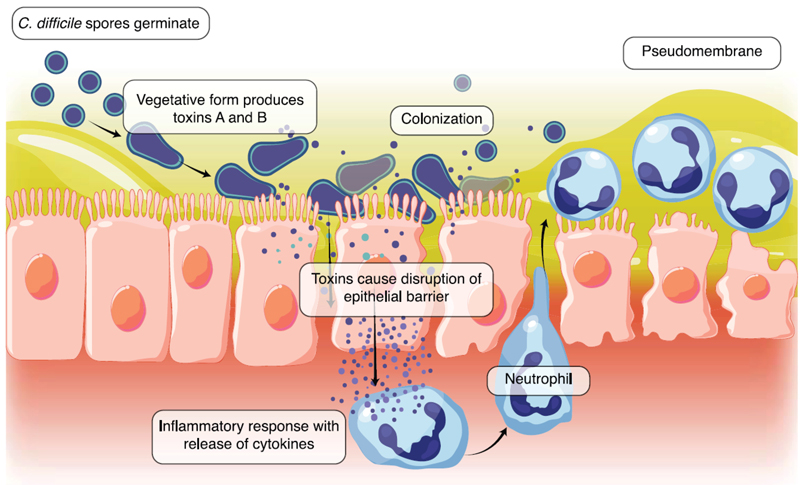 |
| Figure 5. The pathogenesis of Clostridioides difficile. C. difficile spores germinate into toxin-producing vegetative cells, disrupting the intestinal epithelial barrier and resulting in a host inflammatory response. Escalation of the inflammatory response with neutrophil influx results in the formation of a pseudomembrane.31 |
More than 100 C. difficile ribotypes (strains distinguished using a polymerase chain reaction [PCR]-based method) have been identified. Ribotype 027 is notable for its increased toxin production, which is associated with more severe disease. This ribotype has also been strongly linked to the use of fluoroquinolones and the development of fluoroquinolone resistance. Ribotype 027 became particularly prevalent in the 2000s.3,10
Recently, the prevalence of various strains of C. difficile has changed. Ribotype 027 has decreased in frequency, likely due to reduced use of fluoroquinolones and broader efforts to prevent HAIs. As of 2017, despite this decline, ribotype 027 remained the most common ribotype associated with health care settings, accounting for 15% of isolates. In contract, ribotype 106 emerged as the most prevalent ribotype in community-associated cases.4
The standard treatment for CDI typically involves antimicrobial therapy. The most recent clinical guidelines from the American College of Gastroenterology (ACG) recommend using either vancomycin or fidaxomicin as first-line treatment for both severe and nonsevere primary CDI.2 Metronidazole is now reserved for very low-risk patients with nonsevere CDI.
Similarly, the latest guidelines from the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) also recommend fidaxomicin, which is a narrow-spectrum antibiotic approved by the FDA in 2011 for the treatment of CDI.33 This recommendation is based on data demonstrating that fidaxomicin has less activity against other enteric bacteria and is associated with lower rates of CDI recurrence compared with vancomycin.31
Monoclonal antibodies (mAbs) designed to inactivate C. difficile toxins have been developed. Bezlotoxumab, a mAb targeting TcdB, received FDA approval in 2016 for the prevention of recurrent CDI.31 According to the latest IDSA/SHEA guideline, updated in 2021, bezlotoxumab is recommended to be used alongside antibiotic treatment in patients with rCDI.33 This recommendation is based on evidence showing that this combination can reduce recurrence rates. However, it is important to note that the manufacture of bezlotoxumab was discontinued in January 2024.34
Similar to standard antibiotic treatments, mAbs do not address dysbiosis,31 which can create a vicious cycle. In this cycle, antibiotic treatment may exacerbate dysbiosis, allowing C. difficile to reemerge and necessitating additional antibiotic treatment.3,10,25,31 Notably, between 15% and 30% of patients who initially respond to antibiotic treatment will go on to experience rCDI.35
The pathophysiologic processes in rCDI, including changes in gut microbiota, are complex. Most recurrences are caused by the same strain of C. difficile that led to the initial infection; however, some patients with rCDI are infected by different strains, suggesting that underlying dysbiosis makes patients more susceptible to reinfection.10
Restoring gut microbiota to a healthy state (eubiosis) after disruption caused by antibiotic use is possible, but this process may take months or years and may not be completely successful. Research shows that patients remain at risk for rCDI for at least 3 months after the completion of antibiotic therapy for CDI.31
Given these challenges, there is an increasing need for alternative strategies.2,33,36 The dilemma of using antibiotics to treat CDI—while simultaneously increasing the risk for recurrence—has led to a growing interest in microbiota restoration as a preventive strategy. Fecal microbiota-based therapies, which aim to restore healthy composition of gut microbiota and enhance colonization resistance, include fecal microbiota transplantation (FMT) and donor-derived microbial therapeutic products.31 Since 2022, the FDA has approved 2 such microbial products, significantly changing the landscape for rCDI treatment (Table 1).16,36-39
| Table 1. FDA-Approved and Investigational Fecal Microbiota-Based Therapies for CDI and rCDI | |||||
| Therapy | FDA approved? | Guideline inclusion | Contains | How administered | Selected clinical studies |
|---|---|---|---|---|---|
| Conventional fecal microbiota transplant | No |
| Donor stool, screened for pathogens | Multiple methods (eg, enema, colonoscopic, nasogastric/ jejunal tube) |
|
| Fecal microbiota, live-jslm (Rebyota; previously RBX2660) | Yes, for prevention of CDI recurrence in those ≥18 years of age, after antibiotic treatment for rCDI | For prevention of rCDI after ≥2 recurrences or in certain high-risk patients: AGA 2024 | Broad consortium derived from donor stool; standardized for microbial content and screened for pathogens |
|
|
| Fecal microbiota spores, live-brpk (Vowst; previously SER-109) | Yes, for prevention of CDI recurrence in those ≥18 years of age, after antibiotic treatment for rCDI | For prevention of rCDI after ≥2 recurrences (≥3 episodes) or in certain high-risk patients: AGA 2024 | Live, purified spores derived from the stool of qualified, screened donors, standardized for Firmicutes content |
|
|
| VE303 | No | — | Consortium of 8 well-characterized commensal strains of Clostridium species | Oral |
|
| NTCD-M3 (also known as VP20621) | No | — | Spores of a single, nontoxigenic C. diff strain | Oral | Phase 2: NCT01259726 |
| CP101 | No | — | Full-spectrum microbiota derived from donor stool | Oral |
|
| RBX7455 | No | — | Donor stool-derived, room temperature-stable microbiota suspension | Oral | Phase 1: NCT02981316 |
| ADS024 (previously ART24) | No | — | Single strain of Bacillus velezensis | Oral | Phase 1: NCT04891965 |
| Microbial Ecosystem Therapeutic-2 | No | — | Consortium of 40 highly purified microbial strains | Oral | Phase 1: NCT02865616 |
| ACG, American College of Gastroenterology; AGA, American Gastroenterological Association; C. diff, Clostridioides difficile; CDI, Clostridioides difficile infection; IDSA, Infectious Diseases Society of America; rCDI, recurrent Clostridioides difficile infection; SHEA, Society for Healthcare Epidemiology of America. | |||||
Clinical Case 1

What would be appropriate next steps for Ben after his antibiotic treatment?
The earliest microbiota-based therapy introduced for rCDI in clinical practice is FMT. This therapy involves administering stool from healthy donors to restore the GI microbiota of patients with CDI. Typically, FMT is given after the completion of antibiotic treatment.31 The primary goal of FMT is to reestablish a healthy gut microbial community, thereby enhancing resistance to colonization by C. difficile and reducing the risk for future infection.16,31
Although FMT is not FDA-approved, it is permitted under FDA enforcement discretion when treating rCDI unresponsive to standard therapy. The 2021 ACG clinical practice guidelines consider it safe and effective for preventing rCDI.2
FMT can be administered through various methods, including enema, colonoscopy, and nasogastric or nasojejunal tubes. Colonoscopic administration allows for a wider distribution of donor stool throughout the colon, presenting an advantage over enemas. However, this method is more invasive and carries the risks associated with colonoscopy, such as bowel preparation complications, perforation, and sedation-related issues.35,40 FMT administered via nasogastric or jejunal tubes or gastroduodenoscopy carries an increased risk for aspiration and the potential for small bowel bacterial overgrowth.35,40
A randomized, double-blind trial involving 46 patients with 3 or more recurrences of CDI found that 90.9% of those who received colonoscopically administered donor stool achieved resolution of diarrhea without further treatment during an 8-week follow-up.41 In comparison, 62.5% of those who received their own stool (autologous FMT) experienced similar outcomes. Importantly, no serious treatment-related adverse events (TRAEs) were observed.
In another open-label clinical trial conducted in Denmark, patients with rCDI were randomized to receive 1 of 3 treatments: vancomycin (n=16), fidaxomicin (n=24), or vancomycin followed by FMT (n=24), administered by colonoscopy (in 19 patients) or by nasojejunal tube (in 5 patients).42 CDI recurrence within 26 weeks was observed in 81% of patients treated with vancomycin, 51% of those treated with fidaxomicin, and only 13% of those who received vancomycin followed by FMT. All patients with rCDI received vancomycin along with rescue FMT. Successful treatment was associated with improvements in HrQoL scores, which were measured using the EuroQol 5-Dimension 3-Levels questionnaire. Scores improved from 0.675 at baseline to 0.813 at 8 weeks and 0.773 at 26 weeks after initial treatment.42
Open-label trials have their limitations. The recent double-blind, placebo-controlled MATCH study did not find any advantages for FMT in preventing rCDI.43 In this study, 153 adult US veterans with 1 or more episodes of rCDI were randomized to receive either 5 placebo capsules or 5 FMT capsules (manufactured by the Microbiota Therapeutics Program at the University of Minnesota). Participants took the capsules in one sitting, lasting no more than 90 minutes. The FMT capsules contained 5×1011 freeze-dried microbes isolated from donor fecal material. The study was stopped for futility after results showed that 32.9% of the FMT group and 29.9% of the placebo arm experienced clinical recurrence or death within 56 days. Strengths of the study included its size and geographic diversity, with patients from 47 different facilities across 40 US states. Limitations included an overwhelmingly white (94.6%) and male (86.2%) participant cohort.43
Conventional FMT, which uses donor stool often sourced through stool banks, has been practiced in the United States for several decades. Early applications raised safety concerns regarding the potential transmission of infections other than C. difficile. Since then, advanced safety protocols have been implemented, and the FDA has issued guidance requiring stool banks to operate under Investigational New Drug requirements to ensure the safety of FMT materials.44 Nevertheless, the regulatory framework for conventional FMT continues to evolve.45,46
The Fecal Microbiota Transplantation National Registry, maintained by the American Gastroenterological Association (AGA), is tracking the short- and long-term safety of FMT and other microbiota-based therapies, with more than 1000 patients enrolled as of August 2024.47
Fecal microbiota, live-jslm (Rebyota; previously known as RBX2660), received FDA approval in November 2022 for the prevention of rCDI in adults aged 18 years and older after antibiotic treatment for recurrent infection. This marked a significant milestone as it is the first fecal microbiota product derived from human donors to gain FDA approval.16,36
Rebyota is a broad-consortium product made from whole donor stool that has undergone testing for a variety of pathogens and is standardized to contain a defined number of microbes, including >1×105 CFU/mL of Bacteroides.38,48 It is administered rectally via a retention enema in a single 150-mL dose, given 24 to 72 hours after completing antibiotic therapy for the rCDI episode. No bowel preparation is required before administration.38
The approval of Rebyota was based on findings from the PUNCH CD3 randomized, double-blind, placebo-controlled phase 3 trial.48 The primary end point was the absence of CDI-related diarrhea for 8 weeks after treatment. This was analyzed using a Bayesian model that also incorporated data from a previous phase 2 placebo-controlled study. In this trial, adults with at least a single CDI recurrence were randomized in a 2:1 ratio to receive either Rebyota (n=180) or placebo (n=87). The estimated treatment success rates were 70.6% for Rebyota compared with 57.5% for placebo, with a posterior probability of 0.991 favoring the active treatment. In both groups, more than 90% of patients who were recurrence-free at 8 weeks remained so at 6 months of follow-up. The most common side effects associated with Rebyota were abdominal pain and diarrhea. No serious treatment-emergent AEs (TEAEs) were reported.48
The larger, open-label PUNCH CD3-OLS study reported its final results in January 2025.49 This study primarily assessed the safety of Rebyota in adults with rCDI, including older adults and those with concomitant IBD, mild to moderate immunocompromise, and complex comorbidities. Of 793 participants enrolled, 697 received the treatment. Overall, the therapy was well tolerated; most TEAEs were mild to moderate GI disorders, affecting 29.4% of participants. Serious TEAEs occurred in 3.9% of recipients within 8 weeks, predominantly related to underlying conditions or rCDI itself. Efficacy outcomes were consistent with those in PUNCH CD3: Among the modified intention-to-treat population, 73.8% of 676 patients achieved treatment success at 8 weeks, and 91% of these remained recurrence-free at 6 months.49
A subgroup analysis from the PUNCH CD3-OLS study found that mildly to moderately immunocompromised patients (n=141) experienced similar treatment efficacy to non-immunocompromised patients (n=556), with 75.7% and 73% achieving treatment success at 8 weeks, respectively.50 Serious TEAEs occurred in 4.3% of immunocompromised and 3.8% of non-immunocompromised participants, with no new safety signals identified in the immunocompromised group. Importantly, no treatment-related systemic infections were reported. Among the immunocompromised patients, 58.2% had medication-related immunosuppression, 29.1% had an underlying immunocompromising condition, and 12.8% had both.50
In a patient survey assessing perspectives on live biotherapeutic products, the majority of respondents who received Rebyota via rectal administration found the process easy (96%) and quick (94%).51
The colonoscopic administration of Rebyota has been explored in several investigations. However, it is important to note that the FDA indication and labeling for this product specify rectal administration only. A retrospective analysis published in 2024 examined 10 patients who received colonoscopic Rebyota. The results showed that 8 of 10 patients were recurrence-free at 8 weeks (the other 2 were lost to follow-up) and 6 of these individuals had a sustained response at 6 months.52 Another report from an academic center in 2024 showed a 100% success rate in preventing rCDI at 8 weeks among 22 patients administered Rebyota via colonoscopy, with a sustained clinical response seen in 93.8% of participants up to 24 weeks.53
The single-arm, phase 3b CDI-SCOPE trial is currently investigating the safety and effectiveness of colonoscopic administration of Rebyota in adults with rCDI.54 An 8-week update from CDI-SCOPE was published in April 2025. In the update, 41 individuals diagnosed with rCDI (with an average of 3.2 previous CDI episodes) enrolled in the trial and all received Rebyota via colonoscopy, after an antibiotic washout period and bowel preparation. All 39 individuals who completed the 8-week update were recurrence-free; the remaining 2 individuals withdrew their consent and could not be assessed. Overall, 43.9% of the 39 evaluable participants experienced at least 1 AE by 8 weeks, with 5 treatment-related GI events reported in 4 individuals (9.8%). Physicians administering Rebyota reported a “positive” or “very positive” experience in 90.2% of cases, and all indicated that their patients were “much” or “very much” improved at the 8-week visit.55
Data from the phase 2 and phase 3 clinical trials indicate that Rebyota can restore the gut microbiome and metabolic profiles to a state more closely resembling that of healthy individuals. Participants who responded to these trials showed an increased abundance of Bacteroidia and Clostridia, along with a decreased abundance of Gammaproteobacteria, Bacilli, and Erysipelotrichia. These shifts were associated with significantly higher levels of secondary bile acids and decreased levels of primary bile acids.56
In April 2023, the FDA approved Vowst for the prevention of rCDI in patients aged 18 years and older after antimicrobial treatment for rCDI.37,57 These oral capsules contain live, purified spores derived from the stool of qualified, screened donors, with each capsule containing between 1×106 and 3×107 CFU of Firmicutes spores.39,58 Treatment is initiated 2 to 4 days after completing antibiotic therapy for CDI, after bowel preparation with magnesium citrate and an 8-hour fasting period. Patients take 4 capsules once daily for 3 consecutive days.39,58
The approval of Vowst was based on the results of the phase 3 ECOSPOR III trial. In this study, 182 patients with rCDI who had achieved symptom resolution after standard antibiotic therapy were randomized to receive Vowst or a placebo.59,60 The active treatment demonstrated significantly lower recurrence rates compared with placebo (21% vs 47%) for up to 24 weeks after treatment. AEs were generally mild and included abdominal distention, fatigue, constipation, chills, and diarrhea.59,60
A secondary analysis of ECOSPOR III data showed improved HrQoL among treated patients. At 1 week post-treatment, 49% of participants receiving the active treatment and 27% of those on the placebo achieved at least a 10-point improvement on the disease-specific C. difficile Quality of Life Survey. At 8 weeks, these figures increased to 66% for the treatment group and 48% for the placebo arm.61
The open-label, single-arm ECOSPOR IV trial enrolled 263 adults who had recently completed antibiotic therapy for acute CDI. This cohort included participants from ECOSPOR III who experienced a recurrence, as well as new patients with either their first or subsequent recurrences. The treatment using Vowst was generally well tolerated. Most TRAEs were mild or moderate and GI in nature. One patient with a history of adverse reactions to multiple medications experienced mild facial flushing, elevated temperature, and moderate tightness in the throat and jaw after treatment. Serious AEs occurred in 12.5% of the patients. The recurrence rates for CDI were 8.7% through week 8 (69.6% confirmed and 30.4% imputed) and 13.7% through week 24 (61.1% confirmed and 38.9% imputed).62
Age- and antibiotic-stratified analyses from ECOSPOR III demonstrated that active treatment was associated with lower recurrence rates compared with placebo across all subgroups.59 The relative risks were 0.24 for patients aged <65 years; 0.36 for those aged ≥65 years; 0.41 for both vancomycin and fidaxomicin recipients). Similarly, in ECOSPOR IV, recurrence rates by week 8 remained low across all subgroups: 4% for patients <65 years of age and 13.1% for those ≥65 years; 6.5% for patients with 2 previous CDI episodes and 9.7% for those with 3 or more episodes; 10.8% for men and 7.8% for women; 10.4% for those with a toxin-confirmed episode and 4.3% for those confirmed by PCR alone; and 8.9% for vancomycin recipients and 8.3% for fidaxomicin recipients.62
Engraftment of the bacterial species present in Vowst was observed as early as week 1 in treated participants in ECOSPOR III. Compared with those who received a placebo, treated individuals exhibited an increase in Firmicutes bacteria, a decrease in proinflammatory Enterobacteriaceae, and a more significant rise in secondary bile acids by week 8.59
Several microbiota-based therapies are currently being investigated in clinical trials for the prevention of rCDI (Table 1). These therapies involve either single microbial strains or small groups of well-characterized strains. One such therapy is VE303, an investigational oral product that contains a consortium of 8 well-defined commensal Clostridium species.58,63 These strains were originally isolated from donor stool and are now produced from clonal cell banks.63 Research conducted with healthy volunteers has demonstrated that these strains can colonize to the gut after treatment with vancomycin and lead to increases in microbial taxa and metabolites associated with colonization resistance, including short-chain fatty acids and secondary bile acids.64
VE303 was evaluated in the double-blind, placebo-controlled phase 2 CONSORTIUM study, which enrolled 79 adults at high risk for recurrence after primary CDI or with at least 1 recurrence.63 Results published in April 2023 showed recurrence rates through week 8 of 13.8% for the high-dose VE303 group (8.0×109 CFUs), 37% for the low-dose group (1.6×109 CFUs), and 45.5% for the placebo arm. AEs were primarily GI in nature and most common in the high-dose group. Seven participants (4 from the high-dose group, 2 from the low-dose group, and 1 from the placebo arm) discontinued treatment due to diarrhea. No serious AEs were considered related to the study treatment. VE303 is proceeding to phase 3 clinical trials.63,65
Other microbiota-based therapies currently in development include:
- NTCD-M3: This oral product is based on a single, nontoxigenic strain of C. difficile and was evaluated in a phase 2 trial involving 173 adults with primary CDI or a first recurrence.66 The treatment was well tolerated, with recurrence rates of 11% in the NTCD-M3 group compared with 30% in the placebo group.
- Microbial ecosystem therapeutic-2 (MET-2): An oral consortium of 40 highly purified microbial strains, MET-2 was assessed in a phase 1, open-label, single-group trial involving patients with rCDI.67 No serious AEs were reported, and 84% of the 19 treated patients remained recurrence-free through day 130. The strains are independently manufactured and are no longer reliant on stool donors.
- ADS024: This investigational therapy comprises a single strain of Bacillus velezensis and has been studied in a phase 1 trial aimed at preventing rCDI.68
Other broad-spectrum microbiota-based products are currently in clinical development.
- CP101: This oral, full-spectrum fecal microbiota-based therapy was evaluated in a double-blind, randomized, placebo-controlled phase 2 trial involving 198 adults who had experienced at least 1 prior recurrence.69 At 8 weeks, 74.5% of participants receiving CP101 remained recurrence-free compared with 61.5% of those receiving placebo. The treatment was well tolerated, with similar AEs occurring in both groups. Despite these findings, clinical trials of CP101 were halted by the manufacturer in January 2023 following a strategic shift.70
- RBX7455: This orally administered, room-temperature–stable live biotherapeutic product was studied in a phase 1 trial involving 30 adults who had experienced at least 1 CDI recurrence.71 No serious TRAEs occurred, and 90% of treated individuals remained recurrence-free at 8 weeks.
In March 2024, the AGA published updated clinical practice guidelines regarding the use of both FDA-approved and non–FDA-approved (conventional) fecal microbiota-based therapy options for CDI and other GI diseases.16 According to these guidelines, the AGA recommends using fecal microbiota-based therapies to prevent recurrence in immunocompetent adults after they complete antibiotic treatment for rCDI.
The treatment options outlined in the guidelines include Rebyota, Vowst, and conventional FMT. These therapies are recommended for patients experiencing a second recurrence of CDI and for specific individuals who are at high risk for recurrence or severe complications. High-risk patients may include those with a history of severe or fulminant CDI, treatment-refractory CDI, or significant comorbidities.16
The AGA guideline panel has supported the use of conventional FMT as a viable option for preventing rCDI in patients with mild to moderate immunocompromise. However, the panel conditionally advised against using microbiota-based therapies in individuals with severe immunocompromise, unless they are involved in clinical trials. In addition, conventional FMT is recommended as an adjunctive treatment for hospitalized patients with severe or fulminant CDI that does not respond to antibiotic therapy.16
The panel advised caution when prescribing microbiota-based therapies to patients who require frequent antibiotic treatment or long-term prophylactic antibiotics, as these therapies may diminish the efficacy of microbiota-based interventions.
The 2024 AGA guidelines recommend administering Rebyota and Vowst according to their FDA-approved product labeling. Conventional FMT can be delivered through various methods, including nasoenteric tube, colonoscopy, or enema.16
The most recent ACG guidelines on CDI, published in 2021, recommend FMT to prevent further occurrences in patients who have experienced a second or subsequent CDI recurrence. These guidelines also support the use of FMT for preventing CDI recurrences in patients with IBD and rCDI.2 Administration via colonoscopy or oral capsules is preferred; if these methods are unavailable, enema administration is recommended. If recurrence occurs within 8 weeks of the initial administration, a repeat FMT may be performed.2 The 2021 update to the IDSA/SHEA guideline recommends FMT as an option for patients who experience a second or subsequent CDI recurrence, but only after appropriate antibiotic therapy has been attempted for at least 2 recurrences.33
Importantly, the ACG and IDSA/SHEA guidelines were published before the FDA approval of Rebyota and Vowst.
Clinical Case 2

Marina is a 61-year-old woman with a medical history that includes hypertension and mild asthma. Four months ago, Marina was treated with a course of antibiotics for community-acquired pneumonia. However, 1 week later, she developed acute-onset diarrhea and was diagnosed with CDI based on a positive nucleic acid amplification test and a positive toxin enzyme immunoassay. Her symptoms resolved after a course of vancomycin treatment.
Unfortunately, 6 weeks later, her symptoms returned, leading to a diagnosis of rCDI. She received another course of vancomycin, which successfully resolved her symptoms. However, 1 week ago, her symptoms recurred. Currently, Marina is undergoing a 10-day course of vancomycin treatment for rCDI.
What would be appropriate next steps for Marina given her clinical scenario?
Rebyota is indicated for the prevention of rCDI in individuals aged 18 years or older after a course of antibiotics for CDI. It is not indicated for the treatment of active CDI.38
The product is administered rectally as a single 150-mL dose, 24 to 72 hours after the final antibiotic dose, to allow sufficient clearance of residual antimicrobial activity.38 This timing minimizes selective pressure that could favor C. difficile or other pathogens and promotes a gut environment conducive to the engraftment and colonization of the introduced healthy microbial community.
Before use, Rebyota must be completely thawed by placing the carton in a refrigerator for approximately 24 hours.38 The product is supplied with an administration set, and detailed instructions for preparation and administration are provided in the prescribing information. Rebyota does not require patient fasting, bowel preparation, or anesthesia prior to administration. The patient should be positioned in the left lateral or knee–chest position. The administration tube is inserted approximately 5 inches into the rectum, and the product is delivered via gravity flow after unclamping the tubing. After administration, patients are advised to remain in the left lateral position for at least 15 minutes to help minimize cramping. The procedure takes about 5 minutes to complete and can be performed by any qualified health care professional in a variety of care settings.38,72
Rebyota is derived from human fecal material. Although the product undergoes extensive pathogen and contaminant testing, it may contain trace amounts of food allergens and carries a theoretical risk of transmitting infectious agents. As with all biologic products, administration should occur in clinical settings equipped to manage potential hypersensitivity reactions. (Table 2). The only contraindication for this product is a severe allergic reaction (eg, anaphylaxis) to any of its components.
| Table 2. Logistical Considerations for FDA-Approved Microbiota-Based Therapies38,3 | ||
| Fecal microbiota, live-jslm (Rebyota) | Fecal microbiota spores, live-brpk (Vowst) | |
|---|---|---|
| Storage |
|
Stored at 2° to 25°C (36°-77°F) |
| Timing of administration | 24 to 72 hours after the final dose of antibiotic treatment for CDI | 2 to 4 days after the final dose of antibiotic treatment for CDI |
| Bowel preparation | None |
|
| Administration method |
|
|
| Contraindications | Severe allergic reactions (eg, anaphylaxis) to any component of the product | None |
| Warnings and Precautions |
|
|
| Patient counseling information |
|
|
| CDI, Clostridioides difficile infection. | ||
The most commonly reported adverse reactions reported in clinical trials include abdominal pain (8.9%), diarrhea (7.2%), abdominal distension (3.9%), flatulence (3.3%), and nausea (3.3%). Additionally, patients should avoid oral antibiotic therapy for 8 weeks after administration unless specifically directed by their physician.38
Vowst is similarly indicated for the prevention of rCDI in adults after antibiotic treatment and not for the treatment of active CDI. It is administered 2 to 4 days after the completion of antibiotic therapy.39
For bowel preparation, patients should consume 296 mL of magnesium citrate on the day before the first dose, ensuring it is taken at least 8 hours before administration. Alternative regimens are available for patients with impaired renal function. Patients should fast for at least 8 hours before administration of Vowst, with the exception of small amounts of water. Each dose consists of 4 capsules taken once daily for 3 consecutive days, ideally on an empty stomach and before the first meal of the day (Table 2).39
This product may carry a potential risk for transmitting infectious agents and could contain food allergens. Although no specific contraindications have been identified, it is advisable to avoid the concurrent use of antibacterials during the treatment period. The most commonly reported adverse reactions reported in at least 5% of participants included abdominal distension (31.1%), fatigue (22.2%), constipation (14.4%), chills (11.1%), and diarrhea (10%).39
Recurrent CDI remains a significant public health challenge that can be difficult to manage. To improve patient outcomes, a multidisciplinary approach and innovative therapeutic strategies are essential. The robust efficacy and safety outcomes demonstrated in randomized clinical trials for both Rebyota and Vowst support the inclusion of these therapies in recently updated clinical practice guidelines for preventing rCDI. This also justifies their incorporation into treatment formularies. Ongoing research into additional microbiota-based treatment options and novel administration methods may further expand access to these therapies for patients in whom current FDA-approved delivery routes (oral or rectal) are not ideal.73
Considering logistical factors is essential to ensure that patients have access to and can afford microbiota-based therapies. It is important to integrate these therapies with existing systems, such as electronic medical records, billing and insurance processes, and pharmacy workflows, to facilitate their successful implementation.74 Furthermore, the product storage requirements and logistics of administration, including the timing, location, and method of delivery, need to be considered. Both FDA-approved therapies and conventional FMT can be administered in outpatient settings.
The FDA-approved products for treating CDI have specific logistical considerations. Rebyota is administered as a single rectal dose 1 to 3 days after completing antibiotic therapy for CDI. This treatment does not require bowel preparation. It is stored in an ultracold freezer and should be thawed in a refrigerator for approximately 24 hours before administration. If an ultracold freezer is not available, the product may instead be stored in a refrigerator for up to 5 days, inclusive of thawing time. In such cases, facilities should ensure coordination between product ordering and the scheduled administration date to maintain proper storage conditions. Vowst can be stored at standard refrigeration temperatures (36°F-77°F), and is administered as 4 oral capsules taken once daily for 3 consecutive days. However, this treatment requires bowel preparation before the first dose. (Table 2).75
A cost-effectiveness model presented at Digestive Disease week 2025 estimated that both Rebyota and Vowst would be cost-effective for the prevention of rCDI when used after a first recurrence.76 The therapies were projected to save $9274 and $9393, respectively, while providing quality-adjusted life year (QALY) gains of 0.03 and 0.088 compared with placebo. When administered after a second recurrence, Rebyota and Vowst were projected to save $9015 and $11,423, respectively, with corresponding QALY gains of 0.036 and 0.037 compared with placebo.76
Finally, recent real-world investigations are adding to the body of knowledge on practical considerations for FMT in CDI. A Danish cohort study in 1170 patients with rCDI identified several factors that influenced treatment success, including repeat FMT in initial nonresponders (8-week cure rates were 60% after 1 FMT treatment and 81% after up to 5 FMT treatments) and greater efficacy with administration via oral or colonoscopic vs nasogastric or enema routes.77 Of note, this study also found higher cure rates in patients who received more prolonged antibiotic pretreatment with vancomycin and fidaxomicin (45% with 1-5 days of antibiotic pretreatment and 65% with more than 30 days). In contrast, an investigation into the factors leading to treatment failure of conventional FMT identified exposure to non–CDI-specific antibiotics both before and after FMT administration (particularly aminoglycosides and aztreonam), as a significant risk factor for recurrence within 8 weeks. Although the study was conducted at a single center in Korea and did not include any of the FDA approved microbiota-based products, it suggests that avoiding nonessential antibiotic use both before and after microbiota-based treatment is important for promoting success.78
Recurrent CDI presents a significant clinical challenge that imposes heavy burdens on both patients and the health-care system. Two microbiota-based therapies have received FDA approval for preventing CDI recurrence in adults after antibiotic therapy for rCDI. These therapies, along with conventional FMT, have been included in the most recent clinical practice guidelines addressing CDI. Ongoing research studies are providing more information on the efficacy and safety of microbiota-based therapies for preventing rCDI, including their efficacy and safety in various patient subgroups and when administered through different methods. Furthermore, additional research is exploring investigational microbiota-based therapies.
Commentary

“I think it’s important to discuss ahead of time whether it’s likely that the person with CDI will get another infection requiring antibiotic therapy. Examples include people with recurring urinary tract infections, who should have these addressed before proceeding to microbiota-based therapies and those who know they will soon need a joint surgery, for which antibiotics are given preventively. It’s best to provide low-dose vancomycin to these patients until they can have the microbiota-based therapies without getting subsequent systemic antibiotics.
"We have found it very easy to arrange for patients to get the Rebyota either in our clinic or at home. It is sometimes logistically easier than trying to coordinate the pills, which patients must get by mail rather than heading to the local pharmacy."
- Reveles KR, Yang M, Garcia-Horton V, et al. Economic impact of recurrent Clostridioides difficile infection in the USA: a systematic literature review and cost synthesis. Adv Therap. 2023;40(7):3104-3134.
- Kelly CR, Fischer M, Allegretti JR, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol. 2021;116(6):1124-1147.
- Centers for Disease Control and Prevention. 2019 Antibiotic resistance threats report. Updated December 2019. Accessed June 24, 2025. https:www.cdc.gov/antimicrobial-resistance/data-research/threats/?CDC_AAref_Val=//www.cdc.gov/drugresistance/biggest-threats.html
- Guh AY, Mu Y, Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. New Engl J Med. 2020;382(14):1320-1330.
- Feuerstadt P, Theriault N, Tillotson G. The burden of CDI in the United States: a multifactorial challenge. BMC Infect Dis. 2023;23(1):132.
- Magill SS, O’Leary E, Janelle SJ, et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med. 2018;379(18):1732-1744.
- Centers for Disease Control and Prevention. Emerging infections program healthcare-associated infections–community interface report Clostridioides difficile infection surveillance, 2022. Accessed June 24, 2025. www.cdc.gov/healthcare-associated-infections/media/pdfs/2022-CDI-Report-508.pdf
- Gupta A, Khanna S. Community-acquired Clostridium difficile infection: an increasing public health threat. Infect Drug Resist. 2014;7:63-72.
- Yang TJ, Patel AA, Singh J, et al. Increasing trends of Clostridium difficile infection in hospitalized young patients: a study of the National Inpatient Sample from 2007 to 2017. Cureus 2022;14(9):e29497.
- Smith AB, Ocana JS, Zackular JP. From nursery to nursing home: emerging concepts in Clostridioides difficile pathogenesis. Infect Immun. 2020;88(7):e00934-19.
- Spigaglia P. Clostridioides difficile and gut microbiota: from colonization to infection and treatment. Pathogens 2024;13(8):646.
- Doll M, Marra AR, Apisarnthanarak A, Al-Maani AS, Abbas S, Rosenthal VD. Prevention of Clostridioides difficile in hospitals: a position paper of the International Society for Infectious Diseases. Int J Infect Dis. 2021;102:188-195.
- Thornton CS, Rubin JE, Greniger AL, et al. Epidemiological and genomic characterization of community-acquired Clostridium difficile infections. BMC Infect Dis. 2018;18:443.
- Cheng YW, Fischer M. Treatment of severe and fulminant Clostridioides difficile infection. Curr Treat Options Gastroenterol. 2019;17(4):524-533.
- Sucher A, Biehle L, Smith A, Tran C. Updated clinical practice guidelines for C difficile infection in adults. US Pharm. 2021;46(12):HS10-HS16.
- Peery AF, Kelly CR, Kao D, et al. AGA clinical practice guideline on fecal microbiota–based therapies for select gastrointestinal diseases. Gastroenterology 2024;166(3):409-434.
- Carlson TJ, Gonzales-Luna AJ, Garey KW. Fulminant Clostridioides difficile infection: a review of treatment options for a life-threatening infection. Respir Crit Care Med. 2022;43(1):28-38.
- Chua HC, Eubank TA, Lee A, et al. Defining fulminant Clostridioides difficile infections: assessing the utility of hypotension as a diagnostic criterion. Open Forum Infect Dis. 2025;12(2):ofaf033.
- Feuerstadt P, Boules M, Stong L, et al. Clinical complications in patients with primary and recurrent Clostridioides difficile infection: a real-world data analysis. SAGE Open Med. 2021;9:2050312120986733.
- Enoch DA, Murray-Thomas T, Adomakoh A, et al. Risk of complications and mortality following recurrent and non-recurrent Clostridioides difficile infection: a retrospective observational database study in England. J Hosp Infect. 2020;106(4):793-803.
- Feuerstadt P, Nelson WW, Drozd EM, et al. Mortality, health care use, and costs of Clostridioides difficile infections in older adults. J Am Med Dir Assoc. 2022;23(10):1721-1728.e19.
- Falcone M, Russo A, Iraci F, et al. Risk factors and outcomes for bloodstream infections secondary to Clostridium difficile infection. Antimicrob Agents Chemother. 2015;60(1):252-257.
- Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015; 372(9):825-834.
- Mitchell BG, Gardner A. Mortality and Clostridium difficile infection: a review. Antimicrob Resist Infect Control. 2012; 1(1):20.
- Feuerstadt P, Stong L, Dahdal DN, Sacks N, Lang K, Nelson WW. Healthcare resource utilization and direct medical costs associated with index and recurrent Clostridioides difficile infection: a real-world data analysis. J Med Econ. 2020;23(6):603-609.
- Lurienne L, Bandinelli PA, Galvain T, Coursel CA, Oneto C, Feuerstadt P. Perception of quality of life in people experiencing or having experienced a Clostridioides difficile infection: a US population survey. J Patient Rep Outcomes. 2020;4(1):14.
- Heinrich K, Harnett J, Vietri J, Chambers R, Yu H, Zilberberg M. Impaired quality of life, work, and activities among adults with Clostridium difficile infection: a multinational survey. Dig Dis Sci. 2018;63(11):2864-2873.
- Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88-92.
- Rodrigues R, Barber GE, Ananthakrishnan AN. A comprehensive study of costs associated with recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2017; 38(2):196-202.
- Scoble PJ, Reilly J, Tillotson GS. Unrecognized costs associated with Clostridium difficile infection: what HCPs often miss. Pharm Times. September 7, 2021. Accessed May 27, 2025. www.pharmacytimes.com/view/unrecognized-costs-associated-with-clostridium-difficile-infection-what-hcps-often-miss
- Seekatz AM, Safdar N, Khanna S. The role of the gut microbiome in colonization resistance and recurrent Clostridioides difficile infection. Therap Adv Gastroenterol. 2022;15:17562848221134396.
- Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539-1548.
- Johnson S, Lavergne V, Skinner AM, et al. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):e1029-e1044.
- US Food and Drug Administration. FDA drug shortages. December 23, 2024. Accessed May 26, 2025. https://dps.fda.gov/drugshortages/discontinuations/bezlotoxumab-injection
- Song JH, Kim YS. Recurrent Clostridium difficile infection: risk factors, treatment, and prevention. Gut Liver. 2019;13(1):16-24.
- Shao T, Hsu R, Hacein-Bey C, et al. The evolving landscape of fecal microbial transplantation. Clin Rev Allergy Immunol. 2023;65(2):101-120.
- US Food and Drug Administration. Fecal microbiota products. Updated June 7, 2023. Accessed June 24, 2025. www.fda.gov/vaccines-blood-biologics/fecal-microbiota-products
- Rebyota (fecal microbiota, live-jslm) prescribing information. Parsippany, NJ: Ferring Pharmaceuticals Inc.; Nov 2022.
- Vowst (fecal microbiota spores, live-brpk) prescribing information. 2025. Bridgewater, NJ: Aimmune Therapeutics, Inc.; Feb 2025.
- Wynn AB, Beyer G, Richards M, Ennis LA. Procedure, screening, and cost of fecal microbiota transplantation. Cureus. 2023;15(2):e35116.
- Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med. 2016; 165(9):609-616.
- Hammeken LH, Baunwall SMD, Dahlerup JF, Hvas CL, Ehlers LH. Health-related quality of life in patients with recurrent Clostridioides difficile infections. Therap Adv Gastroenterol. 2022;15:1-8.
- Drekonja DM, Shaukat A, Huang Y, et al. A randomized controlled trial of efficacy and safety of fecal microbiota transplant for preventing recurrent Clostridioides difficile infection. Clin Infect Dis. 2025;80(1):52-60.
- US Food and Drug Administration. Enforcement policy regarding Investigational New Drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies: guidance for industry. November 29, 2022. Accessed June 24, 2025. www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-regarding-investigational-new-drug-requirements-use-fecal-microbiota
- Barron M. Fecal microbiota transplants: past, present, and future. American Society for Microbiology. February 9, 2024. Accessed February 10, 2025. https://asm.org/articles/2024/february/fecal-microbiota-transplants-past-present-future
- Hoffman DE, Javitt GH, Kelly CR, Keller JJ, Baunwall SMD, Hvas CL. Gut Microbes 2025;17(1):2493901.
- American Gastroenterological Association. AGA FMT national registry reaches 1,000 enrolled. August 16, 2024. Accessed June 24, 2025. https://gastro.org/news/aga-fmt-national-registry-reaches-1000-enrolled/
- Khanna S, Assi M, Lee C, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a Bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs. 2022;82(15):1527-1538.
- Feuerstadt P, Chopra T, Knapple W, et al. PUNCH CD3-OLS: a phase 3 prospective observational cohort study to evaluate the safety and efficacy of fecal microbiota, live-jslm (REBYOTA) in adults with recurrent Clostridioides difficile infection. Clin Infect Dis. 2025;80(1):43-51.
- Alonso CD, Tillotson GS, Bidell MR, et al. Safety and efficacy of fecal microbiota, live-jslm, in preventing recurrent Clostridioides difficile infection in participants who were mildly to moderately immunocompromised in the phase 3 PUNCH CD3-OLS study. Open Forum Infect Dis. 2025;12(4):ofaf117.
- Feuerstadt P, Oneto C, Tillotson G, Van Hise NW. Patient perception of route of rectal administration of live biotherapeutic product for recurrent Clostridioides difficile infection. Patient Prefer Adherence. 2023;17:2153-2159.
- Knapple WL, Yoho DS, Sheh A, Thul J, Feuerstadt P. Retrospective subgroup analysis of fecal microbiota, live-jslm (REBYOTA®) administered by colonoscopy under enforcement discretion for the prevention of recurrent Clostridioides difficile infection. Therap Adv Gastroenterol. 2024;17:17562848241239547.
- Nguyen L, Axelrad J. Efficacy of fecal microbiota, live-jslm administered cecally via colonoscopy vs rectally via enema in preventing recurrent Clostridioides difficile infection. Presented at American College of Gastroenterology Annual Scientific Meeting & Postgraduate Course. October 27, 2024. Philadelphia, PA; P0237.
- ClinicalTrials.gov. A multi-center, single-arm trial exploring the safety and clinical effectiveness of RBX2660 administered by colonoscopy to adults with recurrent Clostridioides difficile infection (CDI-SCOPE). NCT05831189. Updated January 23, 2025. Accessed June 24, 2025. https://clinicaltrials.gov/study/NCT05831189?term=NCT05831189&rank=1
- Khanna S, Yoho D, Van Handel D, et al. Safety and effectiveness of fecal microbiota, live-jslm (REBYOTA®) administered by colonoscopy for prevention of recurrent Clostridioides difficile infection: 8-week results from CDI-SCOPE, a single-arm, phase IIIb trial. Therap Adv Gastroenterol. 2025;18:17562848251339697.
- Orenstein R, Hecht G, Harvey A, Tillotson G, Khanna S. Two-year durability of REBYOTA™ (RBL), a live biotherapeutic for the prevention of recurrent Clostridioides difficile infections. Open Forum Infect Dis. 2023;10(9):ofad456.
- US Food and Drug Administration. FDA approves first orally administered fecal microbiota product for the prevention of recurrence of Clostridioides difficile infection. April 26, 2023. Accessed June 24, 2025. www.fda.gov/news-events/press-announcements/fda-approves-first-orally-administered-fecal-microbiota-product-prevention-recurrence-clostridioides
- Monday L, Tillotson G, Chopra T. Microbiota-based live biotherapeutic products for Clostridioides difficile infection—the devil is in the details. Infect Drug Resist. 2024;17:623-639.
- Feuerstadt P, Louie TJ, Lashner B, et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N Engl J Med. 2022;386(3):220-229.
- Cohen SH, Louie TJ, Sims M, et al. Extended follow-up of microbiome therapeutic SER-109 through 24 weeks for recurrent Clostridioides difficile infection in a randomized clinical trial. JAMA. 2022;328(20):2062-2064.
- Garey KW, Jo J, Gonzales-Luna AJ, et al. Assessment of quality of life among patients with recurrent Clostridioides difficile infection treated with investigational oral microbiome therapeutic SER-109: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2023;6(1):e2253570.
- Sims MD, Khanna S, Feuerstadt P. Safety and tolerability of SER-109 as an investigational microbiome therapeutic in adults with recurrent Clostridioides difficile infection, a phase 3, open-label, single-arm trial. JAMA Netw Open 2023;6(2):e2255758.
- Louie T, Golan Y, Khanna S, et al. VE303, a defined bacterial consortium, for prevention of recurrent Clostridioides difficile infection: a randomized clinical trial. JAMA. 2023;329(16):1356-1366.
- Dsouza M, Menon R, Crossette E, et al. Colonization of the live biotherapeutic product VE303 and modulation of the microbiota and metabolites in healthy volunteers. Cell Host Microbe 2022;30(4):583-598.e8.
- Menon R, Bhattarai SK, Crossette E, et al. Multi-omic profiling a defined bacterial consortium for treatment of recurrent Clostridioides difficile infection. Nat Med. 2025;31(1):223-234.
- Gerding DN, Meyer T, Lee C, et al. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: a randomized clinical trial. JAMA. 2015;313(17):1719-1727.
- Kao D, Wong K, Franz R, et al. The effect of a microbial ecosystem therapeutic (MET-2) on recurrent Clostridioides difficile infection: a phase 1, open-label, single-group trial. Lancet Gastroenterol Hepatol. 2021;6(4):282-291.
- Kunzmann K. Oral biotherapeutic ADS024 safe, tolerable for recurrent C. difficile prevention. Am J Manag Care. October 26, 2023. Accessed June 24, 2025. www.ajmc.com/view/oral-biotherapeutic-ads024-safe-tolerable-for-recurrent-c-difficile-prevention
- Allegretti J, Kelly CR, Louie T, et al. Safety and tolerability of CP101, a full-spectrum, oral microbiome therapeutic for the prevention of recurrent Clostridioides difficile infection: a phase 2 randomized controlled trial. Gastroenterology 2025;168(2):357-366.
- Walter K. Finch halts phase 3 trial for CP101 for recurrent CDI. HCPLive. January 26, 2023. Accessed June 24, 2025. www.hcplive.com/view/finch-halts-phase-3-trial-cp101- recurrent-cdi
- Khanna S, Pardi DS, Jones C, Shannon WD, Gonzalez C, Blount K. RBX7455, a non-frozen, orally administered investigational live biotherapeutic, is safe, effective, and shifts patients’ microbiomes in a phase 1 study for recurrent Clostridioides difficile infections. Clin Infect Dis. 2021;73(7):e1613-e1620.
- Cotto C, Baker K, Fallon E, Rimon S. Fecal microbiota, live-jslm (rbl; Rebyota®) for prevention of recurrent Clostridioides difficile infection: what gastroenterology nurses need to know. Gastroenterol Nurs. 2024;47(5):378-382.
- Kelly CR, Allegretti JR. Review article: gastroenterology and Clostridium difficile infection: past, present, and future. Clin Infect Dis. 2023;77(suppl 6):S463-S470.
- Cha RR, Sonu I. Fecal microbiota transplantation: present and future. Clin Endosc. 2025;58(3):352-359.
- Sarna K. Do data support the use of commercially available fecal microbiota transplantation products in managing recurrent Clostridioides difficile infection? Drug Information Group, University of Illinois, Retzky College of Pharmacy. October 2023. Accessed June 24, 2025. https://dig.pharmacy.uic.edu/faqs/2023-2/october-2023-faqs/do-data-support-the-use-of-commercially-available-fecal-microbiota-transplantation-products-in-managing-recurrent-clostridioides-difficile-infection/
- Berry P, Voth E, Pardi DS, Khanna S. Cost-effectiveness analysis of fecal microbiota spores, live-brpk and fecal microbiota, live-jslm in managing first and second recurrence of Clostridioides difficile infection. Presented at Digestive Disease Week; May 3-6, 2025; San Diego, CA. Mo2004.
- Paaske SE, Baunwall SMD, Rubak T, et al. Clinical management of Clostridioides difficile infection with faecal microbiota transplantation: a real-world cohort study. EClinicalMedicine. 2025;85:103302.
- Park S-H, Lee J-H, Lee S, et al. Factors for treatment failure after fecal microbiota transplantation in Clostridioides difficile infection. Microorganisms 2024;12(12):2539.
false
*You must be logged in to see results data.
- Which of the following statements about C. difficile infection (CDI) and rCDI is true?
correct answer: b.Rationale: Answer b is correct. Between 20% and 35% of patients who experience their first episode of CDI will have a recurrence, typically within 30 days. rCDI can occur regardless of underlying immune functional status. According to CDC surveillance data, community-acquired cases of CDI outnumbered health care-associated cases in 2022, with rates of 62.1 and 54 per 100,000 individuals, respectively. Since 2022, two microbiota-based products have received FDA approval for the prevention of rCDI, representing a paradigm shift in treatment strategy.
- Which of the following is associated with eubiosis and lower risk for CDI recurrence?
correct answer: c.Rationale: Answer c is correct. Whereas primary bile acids promote C. difficile spore germination, secondary bile acids inhibit spore germination and suppress the growth of vegetative C. difficile cells. A diverse gut microbiota acts as an ecological barrier against pathogenic infections by competing for resources and niches, producing antimicrobial substances, and regulating the immune system. Although interindividual variation exists, healthy individuals typically have a gut microbiota dominated by Bacteroidetes and Firmicutes, with Proteobacteria and Actinobacteria present in smaller amounts. The antibiotics most strongly associated with a risk for CDI include clindamycin, cephalosporins, ampicillin, and fluoroquinolones. However, it is important to note that nearly any antibiotic has the potential to induce CDI.
- The most recent clinical guidelines from the American College of Gastroenterology (ACG) recommend using ____ as first-line treatment for severe and nonsevere primary CDI.
correct answer: a.Rationale: Answer a is correct. The most recent clinical guidelines from the ACG recommend using either vancomycin or fidaxomicin as first-line treatment for both severe and nonsevere primary CDI. Metronidazole is now reserved for very low-risk patients with nonsevere CDI. Bezlotoxumab, a monoclonal antibody targeting C. difficile toxin B, received FDA approval in 2016 for the prevention of recurrent CDI; however, the manufacture of bezlotoxumab was discontinued in January 2024.
- Which of the following therapies requires bowel preparation before use?
correct answer: d.Rationale: Answer d is correct. Both colonoscopic administration of FMT and Vowst require bowel preparation. Rebyota is administered rectally and does not require bowel preparation.
- What is the correct FDA-approved indication for Rebyota and Vowst?
correct answer: b.Rationale: Answer b is correct. Rebyota received FDA approval in November 2022 for the prevention of rCDI in adults aged 18 years and older after antibiotic treatment for recurrent infection. This marked a significant milestone as it is the first fecal microbiota product derived from human donors to gain FDA approval. In April 2023, the FDA approved Vowst for the prevention of rCDI in patients aged 18 years and older after antimicrobial treatment for rCDI.
- Which of the following best describes the administration protocol for Rebyota as approved by the FDA?
correct answer: c.Rationale: Answer c is correct. FDA approval for Rebyota specifies rectal administration of a single 150-mL rectal enema 24 to 72 hours after finishing antibiotics, with no bowel prep required.
- Which of the following best characterizes the efficacy and safety outcomes observed in the PUNCH CD3 and PUNCH CD3-OLS trials for Rebyota?
correct answer: c.Rationale: Answer c is correct. In both PUNCH CD3 and PUNCH CD3-OLS, approximately 70% to 74% of patients had successful outcomes at 8 weeks, and more than 90% of those remained recurrence-free at 6 months, including immunocompromised participants. No systemic infections were reported.
- Which of the following investigational microbiota-based therapies for recurrent C. diff infection is furthest along in clinical development, having completed phase 2 trials and advanced to phase 3?
correct answer: a.Rationale: Answer a is correct. VE303 is the only therapy listed that has completed a successful phase 2 trial (CONSORTIUM study) and is now progressing to phase 3. Others, like RBX7455 and ADS024, remain in phase 1, and NTCD-M3 has completed phase 2 but has not yet moved forward.
- A 61-year-old woman develops CDI 1 week after completing antibiotics for community-acquired pneumonia. The diagnosis is confirmed by a positive nucleic acid amplification test and positive toxin enzyme immunoassay. Her symptoms resolve after a 10-day course of oral vancomycin. 6 weeks later, she experiences her first recurrence and is treated again with oral vancomycin, with symptom resolution. One week ago, she develops diarrhea again. She is currently undergoing a third 10-day course of oral vancomycin for this second recurrence. According to current guidelines and available FDA-approved treatments, what is the most appropriate next step following completion of her current vancomycin course?
correct answer: d.Rationale: Answer d is correct. This patient has experienced multiple recurrent CDI episodes and has now undergone 3 full courses of oral vancomycin, which is not aligned with current guidelines. Repeating vancomycin indefinitely is ineffective and guideline-discordant. Metronidazole is no longer recommended for rCDI. At this stage, microbiota restoration therapies are indicated to prevent further recurrences and should be given after completion of antibiotic therapy.
false