0.75 ACPE contact hour
Faculty
Professor of Anesthesiology
Professor of Surgery (Cardiothoracic)
Duke University School of Medicine
Durham, NC
Associate Dean for Research and Graduate Education
Harborview Medical Center
Professor of Emergency Medicine
Adjunct Associate Professor of Bioengineering and Mechanical Engineering
University of Washington
Seattle, WA
Goal
The goal of this program is to educate clinicians about the pathophysiology, diagnosis, and management of fibrinogen deficiencies, including both congenital and acquired forms.
Intended Audiences
The intended audience for this activity comprises anesthesiologists, general surgeons, trauma surgeons, critical care surgeons, and hospital and health-system pharmacists.
Educational Objectives
- Describe the role of fibrinogen in hemostasis.
- Demonstrate an understanding of congenital and acquired fibrinogen deficiency, including their distinct pathophysiologies, clinical manifestations, diagnostic challenges, and implications for targeted therapeutic decision making.
- Discuss the role of diagnostic testing modalities, such as the Clauss assay and viscoelastic testing, in guiding fibrinogen replacement therapy across clinical settings.
- Review current and emerging management strategies for fibrinogen deficiency, including cryoprecipitate, fibrinogen concentrates, and pipeline therapies.
PHYSICIAN ACCREDITATION STATEMENT
This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Global Education Group (Global) and Applied Clinical Education (ACE). Global is accredited by the ACCME to provide continuing medical education for physicians.
PHYSICIAN CREDIT DESIGNATION
Global designates this enduring activity for a maximum of 0.75 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
PHARMACIST ACCREDITATION STATEMENT
Global is accredited by the Accreditation Council for Pharmacy Education (ACPE) as a provider of continuing pharmacy education with Commendation.
PHARMACIST CREDIT DESIGNATION
Global designates this continuing education activity for 0.75 contact hour (0.075 CEU) of the ACPE (Universal Activity Number UAN 0530-9999-25-030-H01-P). This is a knowledge-based activity.
INSTRUCTIONS FOR OBTAINING CREDIT
To receive credit, participants must participate in the activity and complete and pass the post-test with a minimum score of 70%. Additionally, participants will need to complete the evaluation. CME certificates will be sent via email to those successfully completing the activity.
DISCLOSURES OF RELEVANT FINANCIAL RELATIONSHIPS
Global adheres to the policies and guidelines, including the Standards for Integrity and Independence in Accredited CE, set forth to providers by the ACCME and all other professional organizations, as applicable, stating those activities where continuing education credits are awarded must be balanced, independent, objective, and scientifically rigorous. All persons in a position to control the content of an accredited continuing education program provided by Global are required to disclose all financial relationships with any ineligible company within the past 24 months to Global. All financial relationships reported are identified as relevant and mitigated by Global in accordance with the Standards for Integrity and Independence in Accredited CE in advance of delivery of the activity to learners. The content of this activity was vetted by Global to assure objectivity and that the activity is free of commercial bias. All relevant financial relationships have been mitigated.
The faculty have the following relevant financial relationships with ineligible companies:
- Jerrold Levy, MD: Consulting fees (eg, advisory boards): Bayer, Grifols, Octapharma, Werfen
- Nathan White, MD, MS: Consulting fees (eg, advisory boards): Grifols, HemoSonics Inc
The planners and managers have the following relevant financial relationships with ineligible companies:
- The planners and managers at Global have no relevant financial relationships to disclose.
- The planners and managers at ACE have no relevant financial relationships to disclose.
DISCLOSURE OF UNLABELED USE
This educational activity may contain discussion of published and/or investigational uses of agents not indicated by the FDA. Global and ACE do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of any organization associated with this activity. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
DISCLAIMER
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed in this activity should not be used by clinicians without evaluation of patient conditions and possible contraindications on dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
SYSTEM REQUIREMENTS
For technical support, please contact ACE at cmezonesupport@appliedcme.com or 212-957-5300, ext 280.
GLOBAL CONTACT INFORMATION
For information about the accreditation of this program, please contact Global at 303-395-1782 or cme@globaleducationgroup.com.
FEE INFORMATION AND REFUND/CANCELLATION POLICY
There is no fee for this educational activity.
false
*You must be logged in to see results data.
Fibrinogen deficiency presents significant risks for both bleeding (hemorrhagic) and clotting (thrombotic) complications, making it one of the most critical coagulation disorders.1,2 This deficiency can be congenital, which is inherited, or acquired, and is associated with clinical bleeding ranging from mild to severe, depending on the quantity and quality of circulating fibrinogen and the clinical situation.1 The management of congenital fibrinogen deficiency is typically guided by expert opinion and is influenced by the clinical presentation and the individual’s family history.2 In both congenital and acquired cases, human plasma-derived fibrinogen replacement therapy—available as fresh-frozen plasma (FFP), cryoprecipitate, or fibrinogen concentrates (FCs)—is recommended for major bleeding events.2,3 Although cryoprecipitate has traditionally been the primary option for fibrinogen replacement, FCs have increasingly become a focal point in the modern management of these disorders.1-5 Currently, 2 FCs are approved for treating fibrinogen deficiencies, with a third in clinical development.6-8 Understanding the role of fibrinogen in hemostasis and indications for its replacement will enable clinicians to accurately diagnose, assess risks, and tailor therapeutic approaches. Early recognition and targeted interventions are essential for optimizing patient outcomes.
Fibrinogen Synthesis, Structure, and Function
Fibrinogen is a glycoprotein synthesized in the liver that plays a crucial role in hemostasis. It serves as the precursor to fibrin, which is the primary structural component of blood clots. Fibrinogen production is regulated by the expression of 3 genes—FGA, FGB, and FGG. In humans, plasma levels of fibrinogen reach adult levels as early as 10 to 11 weeks of development. In addition to its role in clot formation, fibrinogen facilitates platelet aggregation, modulates inflammation, and contributes to wound healing.9 Understanding the function of fibrinogen and the disorders related to its deficiency is essential for clinicians, as these disorders can lead to significant bleeding and an increased risk for thrombosis.10
Human fibrinogen is a large, dimeric protein composed of 6 polypeptide chains linked centrally by disulfide bonds, forming a trimeric structure. The polypeptide chains are produced by their respective genes (FGA, FGB, and FGG) and designated as Aα, Bβ, and γ, with molecular masses of 66.5 kDa, 52 kDa, and 46.5 kDa, respectively.11 The fibrinogen molecule is assembled in a stepwise manner. Initially, single chains join to form Aα-γ and Bβ-γ complexes. These then combine to create half molecules, which ultimately combine into the final hexameric structure designated as (Aα/Bβ/γ)2.12 The chains are linked together in the N-terminal E domain by 5 symmetrical disulfide bridges, forming a central disulfide knot. This structure contributes to the overall mass of the molecule, which is approximately 340 kDa. The Bβ and γ chains terminate in globular regions designated as βC and γC modules, respectively, which together form the D domain. Meanwhile, the long Aα chains extend into a series of highly flexible repeats that terminate in a globular αC region. The fully formed fibrinogen molecule contains calcium ion binding sites, the binding of which contributes to both its function and stability (Figure 1).11,12
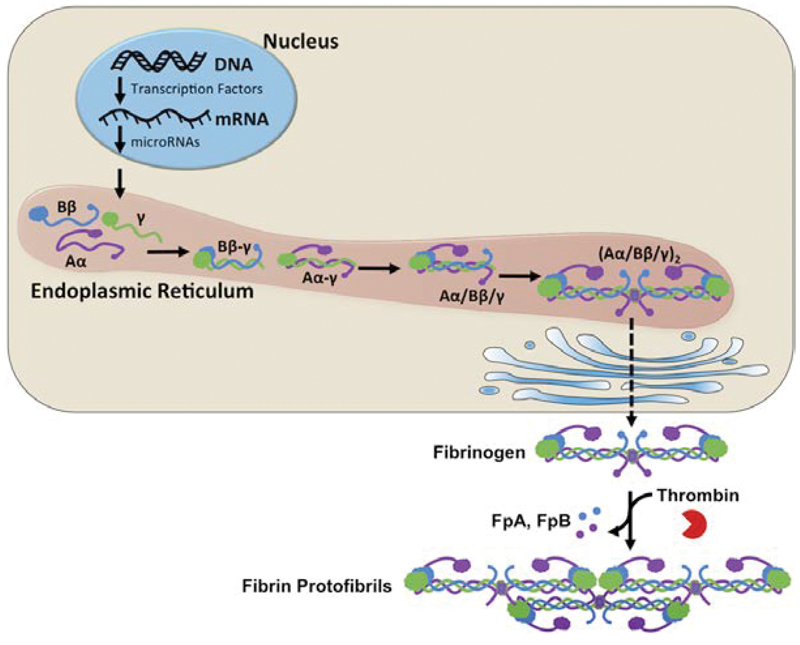 |
| Figure 1. Fibrinogen synthesis and expression. FpA, fibrinopeptide A; FpB, fibrinopeptide B. Reused with permission from Kattula S, et al. Fibrinogen and fibrin in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. |
Fibrinogen plays a key role in hemostasis through several mechanisms.1,9,12
- Conversion to fibrin: During the coagulation cascade, thrombin cleaves fibrinogen, releasing fibrinopeptides. This process allows fibrin monomers to self-polymerize and form a stable clot.
- Platelet aggregation: Fibrinogen acts as a ligand for the platelet integrin αIIbβ3, facilitating the crosslinking and aggregation of platelets. This helps stabilize the hemostatic plug.
- Regulation of fibrinolysis: The degradation of fibrin by plasmin is essential for the dissolution of blood clots. An imbalance in this process can lead to either excessive clotting or excessive bleeding.
The conversion of fibrinogen to fibrin is a crucial step in the coagulation cascade and essential for achieving hemostasis.11 Following tissue injury, the zymogen prothrombin is activated to thrombin, a highly specific serine protease that cleaves small fibrinopeptides from fibrinogen molecules to create fibrin monomers. These monomers spontaneously self-assemble in a staggered, overlapping fashion into an intermediate polymer in the form of 2-stranded protofibrils. Protofibrils further aggregate to form branching fibers that ultimately yield the 3-dimensional insoluble fibrin gel network that provides the structural scaffold of a blood clot.13 Clot formation is further stabilized through fibrin crosslinking via the activation of Factor XIIIa by thrombin. Platelets also contribute to the stabilization of clots in a process known as clot contraction through the binding of fibrinogen via αIIbβ3 (Figure 2).11,12
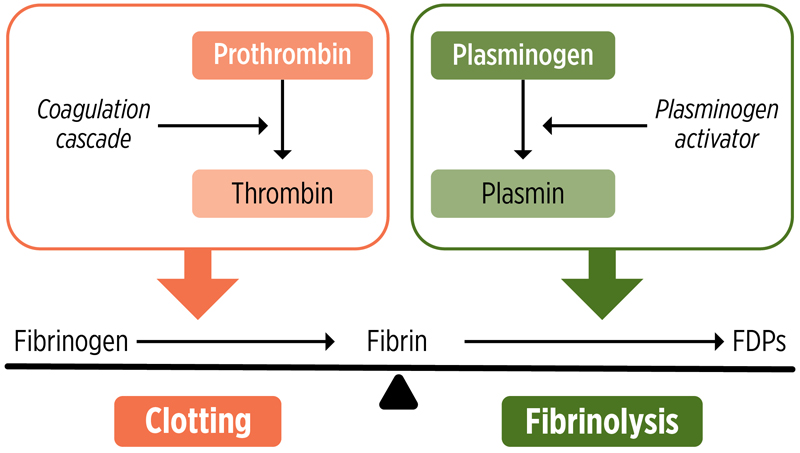 |
| Figure 2. Mechanism of clot formation and lysis. FDP, fibrin degradation product. |
After a clot has formed, it subsequently dissolves under normal conditions through fibrinolysis, allowing for the restoration of blood flow that was impaired during vessel repair and reconstruction.11 For fibrinolysis to occur, plasminogen (Plg) on the fibrin surface must be converted by a Plg activator into the serine protease plasmin. This process results in the enzymatic lysis of fibrin at lysine residues, which dissolves the clot into soluble fibrin degradation products (FDPs). The presence of fibrin accelerates fibrinolysis by forming a cyclic ternary complex of activator, fibrin, and Plg.14 Maintaining a balance between the processes of fibrin formation and fibrinolysis is crucial, as excessive clotting can lead to thrombosis, whereas excessive clot dissolution can result in uncontrolled bleeding.
The clinical manifestations of fibrinogen deficiencies are heterogeneous and depend on the quantity and quality of fibrinogen produced by the individual, as well as the type of disorder (either inherited or acquired).2,15 Presentation can range from a complete absence of symptoms to varying degrees of bleeding (eg, bruising, menorrhagia, miscarriage, epistaxis, gastrointestinal bleeding, and, in rare cases, intracranial hemorrhage). Patients with fibrinogen deficiency may also be at risk for thrombotic events.
Clinically, a fibrinogen level lower than the normal level of 200 mg/dL (2 g/dL) is recognized as an independent risk factor for severe hemorrhage and the need for transfusions in cases of trauma, cardiovascular (CV) surgery, liver transplantation, and gynecologic or obstetric complications.1,4,16 Trauma patients may benefit from an even higher optimal fibrinogen threshold concentration of 2.29 g/L.17 In critical care settings, patients with fibrinogen deficiency frequently present with low fibrinogen levels upon admission. This is associated with poor outcomes, highlighting a major public health concern.2,4,16,18 During CV surgery, fibrinogen levels may decrease further due to the high doses of heparin administered for cardiopulmonary bypass to prevent clot formation, as well as the hemostatic changes associated with extracorporeal circulation. These changes mimic disseminated intravascular coagulation, as evidenced by elevated D-dimers, low fibrinogen levels, increased prothrombin or partial thromboplastin times, thrombocytopenia, and decreased antithrombin levels.4
Fibrinogen levels typically increase significantly during pregnancy, and at delivery correlate directly with the severity of postpartum hemorrhage, bleeding time, the need for invasive procedures, duration of treatment, and timing of intervention.19 Furthermore, patients may experience heavy menstrual bleeding, antepartum bleeding, and miscarriage.1
Timely delivery of treatment can be associated with better outcomes in cases of trauma, CV surgery, major pediatric surgeries, and postpartum hemorrhage. However, FFP and cryoprecipitates must be thawed before administration, which delays their use, or held pre-thawed, which reduces their shelf life.2,16 A randomized controlled trial revealed that trauma patients at risk for bleeding could feasibly receive cryoprecipitate within 90 minutes of hospital arrival, but it demonstrated a median time to administration of 60 minutes, which is of questionable utility during critical bleeding situations.20 These therapies also must be administered in large volumes to ensure delivery of adequate quantities of fibrinogen, and the concentrations of fibrinogen can vary. FCs offer more rapid and specific delivery of a highly purified preparation with a defined fibrinogen content that alters the concentration of other coagulation factors.8,16,21
Congenital fibrinogen deficiencies are rare, accounting for approximately 8% of rare bleeding disorders.2 Mutations in the FGA, FGB, and FGG genes can result in either a quantitative deficiency (eg, afibrinogenemia and hypofibrinogenemia) or a qualitative abnormality (eg, dysfibrinogenemia and hypodysfibrinogenemia).1,9 Patients typically present in early childhood and may experience spontaneous bleeding episodes.1 The prevalence, causes, and presentations of these disorders are summarized in Table 1.1,4,9,22,23
| Table 1. Congenital Fibrinogen Deficiencies: Types, Prevalence, Molecular Causes, and Clinical Presentations1,4,9,22,2 | |||
| Prevalence | Molecular Cause | Clinical Presentation | |
|---|---|---|---|
| Quantitative congenital fibrinogen deficiency | |||
| Afibrinogenemia1,9,22 | ~1-2 cases per | Homozygous or compound heterozygous fibrinogen gene mutations that result in a complete absence of fibrinogen | Severe bleeding manifestations that may include umbilical cord hemorrhage at birth, cutaneous bleeding, hematoma, placental abruption, spontaneous soft tissue bleeding, increased risk for thrombosis, central nervous system bleeding, bone pain, spontaneous splenic rupture, menorrhagia, peritoneal bleeding secondary to hemorrhagic rupture of ovarian cysts, and hemarthrosis |
| Hypofibrinogenemia1,4,9,22 | Variable: 1 per | Commonly heterozygous fibrinogen gene mutations that result in partial fibrinogen deficiency | Often asymptomatic but can lead to bleeding during surgical procedures or after traumatic injuries, miscarriage, postpartum hemorrhage, menorrhagia, placental abruption, fibrinogen storage disease, and risk for thromboses |
| Qualitative congenital fibrinogen deficiency | |||
| Dysfibrinogenemia1,9 | 1 per 100 to 1 per 1000 individuals | Heterozygous missense fibrinogen gene mutations that affect fibrin polymerization | Frequently asymptomatic but can lead to bleeding and/or thromboembolic complications, which include a higher risk for miscarriage, postpartum thrombosis, and menorrhagia |
| Hypodysfibrinogenemia1,23 | Unknown | Heterozygous missense fibrinogen gene mutations that result in reduced levels of fibrinogen with impaired function | Asymptomatic in some cases; more frequently, cutaneous bleeding and mild bleeding associated with obstetric or gynecologic-related hemorrhage or thromboses |
Women with congenital fibrinogen deficiencies are at an overall increased risk for adverse outcomes, beginning with the start of menstruation and continuing through pregnancy and delivery. Menorrhagia is the most prominent symptom in women with afibrinogenemia as they enter reproductive maturity. This condition includes risks for massive hemoperitoneum, which may require surgical intervention, and ruptured corpus luteum cysts during the luteal phase that can lead to oophorectomy.24 According to a systematic review of 188 pregnancies in 70 women between 1985 and 2018, those with congenital fibrinogen deficiencies were at increased risk for obstetric complications, with higher rates of miscarriage during the first trimester (42.9% vs 20%), placental abruption (8% vs 0.5%), and postpartum hemorrhage, compared with the general population (19.4% vs 2%-3%).2,25 Similar rates of miscarriage were observed in a 2024 analysis, with spontaneous abortion occurring in 31% (21 of 68) of pregnancies in women with congenital fibrinogen deficiencies, 86% of which occurred in pregnant women with dysfibrinogenemia and 14% in those with hypofibrinogenemia.1
Acquired fibrinogen deficiencies are more prevalent than congenital cases and can arise from external factors that either disrupt the synthesis or function of fibrinogen, or lead to its consumption.9,26 Common causes and mechanisms that can lead to fibrinogen depletion are summarized in Table 2.2,9,15,26
| Table 2. Acquired Fibrinogen Deficiencies: Types, Causes, and Clinical Presentation2,9,15,2 | ||
| Type | Cause | Mechanism |
|---|---|---|
| Hypofibrinogenemia | Liver disease2,9,15,26 | Reduced liver function resulting in impaired fibrinogen biosynthesis |
| Disseminated intravascular coagulation, sepsis, thrombolytic therapy2,9,15,26 | Consumption coagulopathy, which reduces fibrinogen level and increases FDP level in the plasma, impairing normal fibrinogen function | |
| Massive transfusion9,15,26 | Hemodilution of coagulant factors through the delivery of large volumes of blood that results in a reduced concentration of fibrinogen in the plasma | |
| Dysfibrinogenemia | Major trauma and hemorrhage9,26 | Significant blood loss that triggers reduced levels of fibrinogen in the plasma |
| Autoimmune disease (eg, rheumatoid arthritis, systemic lupus erythematosus), malignancy (eg, myeloma), certain medications (eg, l-asparaginase), and the use of plasma exchange with albumin as replacement fluid2,9,15,26 | Production of anti-fibrinogen antibodies that result in autoimmune clearance of fibrinogen | |
| Liver disease2,9,15,26 | Development of liver disease, including liver failure (eg, chronic liver disease, and cirrhosis) that leads to decreased fibrinogen levels and impaired clot formation due to an increase in sialic acid residue content that interferes with fibrin polymerization | |
| Hyperfibrinolysis26 | Amplified breakdown of fibrinogen into fibrin that disrupts the balance between the formation and degradation of fibrin | |
| FDP, fibrinogen degradation product. | ||
Clinically, acquired fibrinogen deficiencies can manifest symptoms similar to those seen in congenital cases, with some patients exhibiting no symptoms and others presenting with bleeding or thrombotic events.2,15 As with congenital causes, presentation largely depends on etiology and the presence of other coagulation abnormalities.
Due in part to the overlapping clinical symptomatology among disorder subtypes, the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis developed a classification system for congenital fibrinogen deficiencies. The system is based on symptom presentation and severity, as well as fibrinogen levels, and is intended to aid in the diagnosis and differentiation of these deficiencies.27 However, factors that inhibit fibrin formation, as well as those that decrease light transmission, can reduce the accuracy of the results.
For example, in CV surgery, high doses of heparin are often used to prevent thrombosis during cardiopulmonary bypass and extracorporeal circulation.15 Heparin use, along with fibrinogen consumption during extracorporeal circulation, may reduce levels of fibrinogen both during and after surgery, causing acquired hypofibrinogenemia or dysfibrinogenemia, which may increase the risk for bleeding.
Moreover, the rarity of these disorders can complicate the diagnosis of specific subtypes, particularly in the case of hypodysfibrinogenemia. This condition is infrequently reported and shares characteristics with both hypo- and dysfibrinogenemia, potentially contributing to misdiagnoses despite the presence of distinct biological variants, clinical phenotypes, and molecular genotypes.
Currently available FC products are not approved for use in all types of fibrinogen deficiencies (eg, dysfibrinogenemia). Therefore, understanding a patient’s specific subtype of congenital or acquired fibrinogen deficiency has important implications for effective treatment.6,7 As more mutations are identified, genotyping is beginning to play an important role in confirming diagnosis, characterizing subtypes, and guiding treatment selection.1
Another challenge to ensuring the delivery of quality care for patients with fibrinogen deficiency is the variability in treatment recommendations. Existing guidelines are largely based on expert opinion and are influenced by individual patient presentation and family history.2 Although fibrinogen repletion with FC is widely performed to treat bleeding episodes, a consensus on the precise dose of fibrinogen to be administered has not yet been determined.4,28 Various international guidelines from the United States, Great Britain, Europe, and Australia offer recommendations for the administration of FCs to patients with massive hemorrhage-associated coagulopathy, but there is currently no standardized minimum dose or assay for measurement of fibrinogen levels across groups.22,28 Thus, protocols for fibrinogen replacement therapy may vary across institutions depending on the guidelines most familiar to the administering clinicians.
Accurate diagnosis is crucial for effective management of bleeding disorders. Various laboratory tests, including the Clauss assay, viscoelastic testing (VET), thrombin clotting time (TT), and fibrinogen antigen assays, are essential in this effort.26 Clinicians may find it challenging to differentiate between congenital and acquired fibrinogen deficiencies, as the laboratory findings for both types can overlap. A structured diagnostic approach is also essential for accurate differentiation, development of individualized patient management plans, and achievement of optimal clinical outcomes.1,2 Integrating knowledge of the utility of available clinical tests, understanding hemostasis, interpreting laboratory test results, and considering patient history will aid in making an accurate diagnosis and providing effective treatment.
Clauss Assay
The Clauss fibrinogen assay is the most widely used test for evaluating fibrinogen function.1,2,26,28 This assay involves adding a high concentration of thrombin to a diluted plasma sample to measure the clotting time, which has an established inverse relationship with fibrinogen concentration.26 It can be a valuable component of hemostasis testing with reported turnaround times of less than 20 minutes when adapted for critical situations.29 However, it has several limitations. For example, it can yield misleading results in the presence of thrombin inhibitors, such as direct thrombin inhibitors (DTIs), and high levels of FDPs. Moreover, samples from patients receiving unfractionated heparin (UFH) therapy or those collected from heparin-contaminated lines may show falsely low fibrinogen levels. Additionally, the detection of clot formation by turbidity rather than mechanical means can complicate interpretation. Finally, the Clauss fibrinogen assay requires whole blood to be processed to plasma, and this capability may be limited to central laboratory settings rather than available at the point of care.
Viscoelastic Testing
Traditional VETs, which include thromboelastography (TEG) and rotational thromboelastometry (ROTEM), provide real-time assessment of clot formation, clot firmness, and fibrinolysis.2,26,30,31 These point-of-care tests are particularly valuable in emergency settings, such as trauma and cardiac surgery.
The basic parameters studied in TEG include the following30,31:
- R time: This measures the time until initial fibrin formation and depends on the presence of clotting factors. The rapid TEG uses kaolinand tissue factor.
- K (seconds): This indicates the time required to achieve a specific level of clot strength.
- Alpha angle: This parameter assesses the rate of fibrin buildup and crosslinking, which are influenced by fibrinogen levels.
- Maximum amplitude: This represents the overall clot strength, which is affected by both platelets and fibrinogen.
- LY30 (%): This percentage measures fibrinolysis by tracking clot breakdown over a 30-minute period.
ROTEM uses different nomenclature but assesses similar parameters30:
- Clotting time: This uses tissue factor as the activator and is similar to the R value in rapid TEG.
- Alpha angle and clot formation time: These correspond to the K value and alpha angle in TEG.
- Maximum clot firmness (MCF): This is equivalent to the maximum amplitude measured in TEG.
- Clot lysis (LY30): This is the same as the LY30 value used in TEG.
VETs can provide rapid, real-time insights into hemostasis, especially when used in a multiplexed approach to rapidly identify key hemostatic abnormalities. However, VETs also require specialized training, interpretation, and upkeep, which can limit their widespread use.31
Thrombin Clotting Time and Reptilase Time
TT measures the time necessary for a fibrin clot to form after thrombin is added to plasma; it can be performed as the initial screening test for functional fibrinogen.26 Although TT is useful in identifying fibrinogen abnormalities, it has low specificity because various factors can cause TT prolongation. To help differentiate between heparin contamination and anticoagulation caused by fibrinogen deficiency or loss of function, a test called reptilase time may be performed alongside TT. This alternative test uses an enzyme isolated from snake venom and is not affected by heparin.
Fibrinogen Antigen Testing
Fibrinogen antigen assays, commonly performed using enzyme-linked immunosorbent assay (ELISA) or immunoturbidimetric techniques, measure the total amount of antigenic fibrinogen protein in plasma.26 ELISA assays, in particular, provide the best accuracy and sensitivity for samples with low concentrations and can help differentiate between qualitative and quantitative congenital fibrinogen deficiencies.2,27 However, these assays can be time-consuming and are not ideal for routine testing. Additionally, fibrinogen antigen tests do not assess fibrinogen functionality, necessitating further functional tests for comprehensive evaluation.
Prothrombin Time-Fibrinogen Assay
Prothrombin time-fibrinogen (PT-Fg) assays offer an indirect estimate of fibrinogen levels in a platelet-poor plasma sample by comparing the PT of the sample to that from a series of plasma dilutions with known fibrinogen concentrations.2,26 User technique and reagent variability can greatly affect results.
Genotyping
To date, more than 300 causative variants for congenital fibrinogen deficiencies have been identified and recorded in databases, such as the Human Fibrinogen Database.1,26,32 When a congenital fibrinogen deficiency is suspected, genotyping should be considered, if available, as part of the testing process. This can help confirm the diagnosis, differentiate between subtypes, facilitate screening of family members, and enable prenatal evaluation.26
Table 3 summarizes common laboratory tests that may be conducted as part of a stepwise approach to the diagnosis of fibrinogen deficiencies and outlines their expected laboratory values.
| Table 3. Typical Laboratory Testing Outcomes in Congenital and Acquired Fibrinogen Deficiencies | ||
| Test | Congenital | Acquired |
|---|---|---|
| Fibrinogen activity (Clauss method) | Low or undetectable | Low to normal |
| Antigenic fibrinogen levels | Low in quantitative cases, normal in dysfibrinogenemia | Decreased |
| Genetic testing | Confirms mutations in FGA, FGB, FGG | Not applicable |
| D-dimer and FDP levels | Normal or mildly elevated | Markedly elevated (DIC, sepsis) |
| Liver function tests | Normal | Often abnormal (hepatic disease) |
| Viscoelastic testing (TEG/ROTEM) | Impaired clot formation | Reduced clot strength in trauma, DIC |
| DIC, disseminated intravascular coagulation; FDP, fibrin degradation product; ROTEM, rotational thromboelastography; TEG, thromboelastography. | ||
Clinical Utility of Diagnostic Tests
The Clauss test is the most used assay for assessing functional fibrinogen levels in patients with coagulopathy due to massive hemorrhage. However, newer guidelines are starting to recommend the use of newer, more reliable VETs when available.28,33 Despite these developments, there is still no universal consensus on which test should be used in specific clinical situations, and recommendations vary by society. Recent guidelines are outlined in Table 4.33-37
| Table 4. Recent Guideline Recommendations for Functional Fibrinogen Measurement in Patients With Massive Hemorrhage-Associated Coagulopathy33-3 | ||
| Guidelines | Clinical Setting | Test |
|---|---|---|
| European Guideline on Management of Major Bleeding and Coagulopathy Following Trauma 202333 | Bleeding coagulopathy following trauma | Nonguided initial dosage |
| Management of Severe Perioperative Bleeding: Guidelines From the European Society of Anesthesiology and Intensive Care 202334 |
|
|
| Patient Blood Management Guideline for Adults with Critical Bleeding 202335 | Major hemorrhage that is likely to result in massive transfusion | VETs or Clauss assay |
| Society of Cardiovascular Anesthesiologists Clinical Practice Improvement Advisory for Management of Perioperative Bleeding and Hemostasis in Cardiac Surgery Patients 201936 | Perioperative bleeding |
|
| The Use of Viscoelastic Haemostatic Assays in the Management of Major Bleeding: A British Society for Haematology Guideline 201837 | Obstetric, liver, or cardiac surgery | VETs or Clauss assay |
| VET, viscoelastic testing. Adapted from Leal-Noval, et al. Blood Transfus. 2005. |
||
Challenges in Diagnostic Testing
Despite advancements in laboratory testing, several gaps remain in the diagnostic approach to fibrinogen disorders.
- Interference from anticoagulants: Many fibrinogen assays, particularly the Clauss assay, can be affected by anticoagulants such as UFH and DTIs. This interference may lead to misdiagnoses.26
- Limited standardization of VET: Although VET provides real-time information about clot dynamics, its results can vary significantly depending on reagent selection and device calibration. This variability limits its reliability in diagnosing fibrinogen disorders.31
- Overestimation by PT-Fg assay: The PT-Fg assay can overestimate fibrinogen levels in patients with dysfibrinogenemia or those undergoing anticoagulant therapy, which may lead to misclassification of fibrinogen deficiencies.26
- Need for combined testing: No single test comprehensively depicts fibrinogen function. A combination of Clauss assay, antigen testing, and viscoelastic methods may be necessary.1,26,27
- Limited accessibility of genetic testing: Genetic analysis is not widely available in all health care settings.1,26-27
In the future, improvements in diagnostic strategies should focus on enhancing test standardization, increasing accessibility to genetic testing, and developing integrated diagnostic algorithms to achieve better patient outcomes.
Fibrinogen is often the first clotting factor to reach critically low levels during hemorrhagic events, making targeted fibrinogen replacement important.16 Fibrinogen replacement therapy can be administered intravenously using cryoprecipitate or applied topically with liquid adhesives. Although FFP is used clinically, it is not an appropriate source of fibrinogen.4,38 Management strategies for fibrinogen replacement have evolved significantly over time, transitioning from traditional plasma-derived therapies to more targeted solutions.
Cryoprecipitate and FFP
Historically, cryoprecipitate and FFP have been the primary options for fibrinogen replacement in cases of trauma and massive transfusion.2-4 Although both therapies provide essential coagulation factors, plasma-based therapies have notable limitations.2,3,16
- Inconsistent fibrinogen content: Variable potency makes precise dosing challenging.
- Risk for viral transmission: Despite modern screening, some risk remains.
- High-volume requirements: Large infusion volumes can lead to fluid overload, especially in patients with cardiac or renal conditions.
- Delayed administration: Both products require thawing before use, which can cause delays during massive transfusion.
- ABO compatibility: Matching blood types is necessary for safe transfusion.
Although FFP is commonly used to replenish coagulation factors, it is not ideal for fibrinogen repletion due to its low concentration (1-3 mg/mL).3,4 Large volumes, upward of 2 L are required, increasing the risk for fluid overload. In contrast, cryoprecipitate, which is derived from thawed FFP, is a more concentrated source of fibrinogen, along with Factor VIII, von Willebrand factor, Factor XIII, and fibronectin.
Cryoprecipitate is produced by thawing FFP, centrifuging to isolate the insoluble proteins, and then resuspending and refreezing the precipitate.4 Each unit of cryoprecipitate, which typically ranges from 10 to 20 mL, contains approximately 200 to 250 mg of fibrinogen.16 Before transfusion, cryoprecipitate should be typed for blood compatibility. Compared with FFP, cryoprecipitate provides a more concentrated source of fibrinogen but still requires a larger volume than FCs and carries a risk for pathogen transmission.4 Its use in trauma care is inconsistent. In the PROMMTT observational study of 10 US level I trauma centers, administration of cryoprecipitate to severely injured patients was highly variable and often delayed or omitted altogether. Importantly, no association was found between cryoprecipitate use and in-hospital mortality.39
FCs have emerged as a superior alternative to cryoprecipitate and FFP, due in part to the fact that they can be standardized and have undergone virus inactivation.3,40 The key FCs are described below.
RiaSTAP
RiaSTAP (fibrinogen concentrate [human]) is a lyophilized FC derived from human plasma, formulated to treat congenital fibrinogen deficiencies such as afibrinogenemia and hypofibrinogenemia. It facilitates clot formation by rapidly increasing plasma fibrinogen levels.6
This product received orphan drug designation from the FDA in 2008, underscoring its importance for a small but significant population.6,41 In 2009, RiaSTAP became the first FDA-approved treatment for congenital fibrinogen deficiency.41 Approval was based on a prospective, open-label phase 2 study that assessed pharmacokinetics and safety, with ROTEM MCF serving as a key indicator of hemostatic efficacy.6,42 The study showed a significant improvement in MCF (mean change: 8.9 mm; P<0.0001) within 1 hour after infusion, with peak plasma fibrinogen levels achieved within 30 to 60 minutes, and more than 90% of patients meeting hemostatic end points.42
Further clinical trials confirmed the effectiveness of RiaSTAP in various surgical and trauma settings, including aortic surgery, demonstrating that FC significantly reduced the need for allogeneic blood transfusions compared with FFP.43-45 A phase 3 trial is underway to investigate its use for acquired fibrinogen deficiencies.46
A comprehensive 2024 postmarketing safety analysis spanning 35 years and involving 337 patients confirmed a low rate of adverse reactions (806 reported adverse drug reactions with approximately 9243 g administered).47 Thromboembolic events and severe allergic reactions remain the most serious risks.6,47 RiaSTAP is generally well tolerated, with common adverse effects such as rash, fever, headache, and rare cases of thrombosis such as deep vein thrombosis, myocardial infarction, and pulmonary embolism.6,47 It is contraindicated in individuals with severe hypersensitivity to any of its components and is not indicated for dysfibrinogenemia.6 Due to the risk for thrombosis, physicians should carefully evaluate the benefits of administering RiaSTAP to patients with a predisposition to clotting disorders.
RiaSTAP remains stable for up to 60 months when refrigerated,6 offering an extended shelf life for certain clinical settings.
Fibryga
Fibryga (fibrinogen [human]) is a lyophilized FC derived from human plasma and is currently the only FC approved for both congenital and acquired fibrinogen deficiencies.7,48 It was first approved by the FDA in 2017 for treating acute bleeding episodes in adults and adolescents with congenital fibrinogen deficiency. In 2020, this approval was expanded to include pediatric patients younger than 12 years of age.7,48 The landmark 2024 FDA approval for acquired fibrinogen deficiencies further solidifies the role of fibryga as the sole FDA-approved replacement therapy for this condition, making it essential for treating severe bleeding conditions in various clinical settings.7,48
Approval was based on the FORMA-02 and FORMA-04 phase 3 clinical trials.7,49 These multinational, prospective, open-label studies were designed to evaluate efficacy and safety in the prevention of perioperative bleeding in patients with congenital fibrinogen deficiency. The trials demonstrated that fibryga successfully achieved target fibrinogen levels, resulting in a 100% success rate in managing hemostasis across 15 surgeries (13 minor and 2 major surgeries). Among the minor surgeries were dental procedures, such as tooth extractions, root canals, and pulpectomies, as well as soft tissue procedures (eg, skin biopsies and debridement for superficial necrosis), and joint-related interventions, including radioisotope synovectomy. Circumcision and circumcision revisions were also included. The 2 major surgeries included a right eye enucleation with socket reconstruction in an adult and a splenectomy in a child after a spontaneous spleen rupture. After administering preoperative loading doses, plasma fibrinogen levels increased rapidly. For minor surgeries, the average dose required was 74.3 mg/kg in adults, 74.87 mg/kg in adolescents aged 12 years and older, and 91.5 mg/kg in children aged less than 12 years.52 Multiple infusions were required for patients undergoing both major surgeries (8 for the right eye enucleation and 6 for the pediatric splenectomy), with doses reaching 450.4 mg/kg in children. However, blood loss was lower than anticipated, and no transfusions were needed.49 The safety profile was favorable, with no thromboembolic events reported in adults and one case of portal vein thrombosis in a child, which occurred after the splenectomy and is a recognized procedural risk.
The efficacy and safety in acquired fibrinogen deficiencies were demonstrated in the multicenter phase 3 FIBRES study.7,50 This trial confirmed that fibryga was noninferior to standard-of-care (SOC) fibrinogen replacement in reducing intraoperative blood loss while maintaining safety.
Fibryga is generally well tolerated but is contraindicated in patients with severe hypersensitivity to any of its components.7,49,50 Reported adverse effects include allergic reactions such as hives, urticaria, wheezing, hypotension, chest tightness, and anaphylaxis, as well as thromboembolic events like deep vein thrombosis, myocardial infarction, and pulmonary embolism. As with RiaSTAP, physicians should carefully evaluate the benefits of fibryga for patients who are predisposed to clotting disorders, due to the risk for thrombosis. Additionally, this therapy is not indicated for dysfibrinogenemia, a condition characterized by dysfunctional fibrinogen.
Unlike cryoprecipitate or FFP, fibryga can be rapidly reconstituted, provides precisely controlled fibrinogen concentration, can be delivered at the bedside to a patient experiencing bleeding, does not require crossmatching, and has a reduced risk for pathogen transmission due to its advanced purification and virus inactivation processes.3,4,7,49,50 Furthermore, the room-temperature stability of fibryga enhances its practicality for use in trauma, cardiac surgery, and perioperative settings, where precise fibrinogen replenishment and immediate availability are crucial.
BT524
BT524 is an FC developed for the treatment of acquired fibrinogen deficiencies.8,51 It is a purified, plasma-derived product that restores fibrinogen levels in patients experiencing significant blood loss.8 The phase 3 AdFIrst study demonstrated that BT524 was noninferior to SOC cryoprecipitate or FFP in reducing intraoperative blood loss, with a faster time to administration, shorter administration duration, and a mean reduction in blood loss of 279 mL.8 The safety profile was favorable, with significantly fewer thromboembolic events reported in the BT524 arm vs the SOC arm. Additional data on BT524 were presented in June 2025 at the Research and Practice in Thrombosis and Haemostasis conference.
- In a phase 3 study in adults undergoing abdominal surgery, use of BT524 was associated with reduced intraoperative blood loss, increased fibrinogen correction, a favorable safety profile, and fewer thromboembolic events.52
- A phase 3 study in adults undergoing spinal surgery showed reduced intraoperative blood loss, increased fibrinogen correction, and fewer serious adverse effects with the use of BT524.53
Regulatory approval is being pursued for BT524 in both Europe and the United States. If approved, BT524 will become the second FC specifically indicated for acquired fibrinogen deficiencies in the United States. Like other FCs, BT524 carries a potential risk for thromboembolic events, necessitating careful patient selection.
CSL511
CSL511 is an FC currently being investigated in a phase 3 clinical trial involving 90 patients with pseudomyxoma peritonei (PMP) who are undergoing cytoreductive surgery and are expected to experience significant intraoperative blood loss (≥2 L).54 (PMP is a rare cancer characterized by the progressive accumulation of mucinous tumors throughout the abdominal cavity, requiring extensive surgeries that carry a high risk for major bleeding and the need for large-volume blood transfusions.55) This prospective, open-label, randomized study compares the effectiveness of CSL511 vs SOC cryoprecipitate.54
Early identification of low fibrinogen levels and targeted replacement are crucial for achieving hemostasis. Traditional options, such as FFP and cryoprecipitate, although historically effective, have limitations in consistency, safety, speed, and ease of administration. Recent advances in fibrinogen replacement therapy have significantly improved the management of both congenital and acquired fibrinogen deficiencies. FCs enhance treatment by providing rapid, targeted, and pathogen-inactivated solutions.
Case 1: Congenital HypofibrinogenemiaA 16-year-old girl with congenital hypofibrinogenemia (diagnosed by prior bleeding and low Clauss assay, most recently 70 mg/dL) and mild chronic kidney disease (CKD) from a congenital solitary kidney, is scheduled for laparoscopic appendectomy. The standard protocol calls for cryoprecipitate, but none is available on short notice. The nephrology team is concerned about the risk for fluid overload. To address this, the hematology team administers RiaSTAP FC 70 mg/kg, which provides a precise, standardized dose in a minimal volume. After the infusion, Clauss and FIBTEM assays confirm that hemostatic targets have been met. The surgery proceeds uneventfully, and the patient’s renal function remains stable. Key Points
|
Case 2: Severe Postpartum HemorrhageA 27-year-old previously healthy woman undergoes spontaneous vaginal delivery at term. Shortly after delivery, she develops significant postpartum hemorrhage due to uterine atony. Her initial labs show a Clauss fibrinogen level of 1 g/L and ROTEM FIBTEM A5 of 6 mm. She is tachycardic and borderline hypotensive. The blood bank is contacted for cryoprecipitate, but a delay of at least 30 minutes is expected, and the estimated volume required would be high. To enable rapid correction and limit fluid overload, the obstetrics team decides to use fibryga, which is reconstituted and infused in 100 mL, administered in conjunction with established treatments for uterine atony. Within 30 minutes, ROTEM FIBTEM rises to 13 mm, bleeding subsides, and the patient’s hemodynamics stabilize without risk for fluid overload. Key Points
|
- Mohsenian S, et al. Blood Adv. 2024;8(6):1392-1404.
- May JE, et al. Hematol Oncol Clin North Am. 2021;35(6):1197-1217.
- Franchini M, et al. Blood Transfus. 2012;10(1):23-27.
- Levy JH, et al. Blood. 2015;125(9):1387-1393.
- White NJ, et al. J Trauma Acute Care Surg. 2017;82(6 suppl):S41-S49.
- RIASTAP [fibrinogen concentrate (human)] prescribing information. Kankakee, IL: CSL Behring LLC. June 2021.
- FIBRYGA [fibrinogen (human)] prescribing information. Paramus, NJ: Octapharma USA, Inc. Nov 2014.
- Rahe-Meyer N, et al. eClinicalMedicine. 2025;103264: Article in press.
- Vilar R, et al. Haematologica. 2020;105(2):284-296.
- Wolberg AS. J Thromb Haemost. 2023;21:3005-3015.
- Weisel JW, Litvinov RI. Semin Thromb Hemost. 2017;43(3):249-256.
- Kattula S, et al. Arterioscler Thromb Vasc Biol. 2017;37(3):e13-e21.
- Doolittle RF. Adv Protein Chem. 1973;27:1-109.
- Hoylaerts M, et al. J Biol Chem. 1982;257(6):2912-2919.
- Besser MW, MacDonald SG. J Blood Med. 2016;7:217-225.
- Keltner NM, et al. Transfusion. 2024;64(suppl 2):S136-S145.
- Hagemo JS, et al. Crit Care. 2014;18(2):R52.
- Rourke C, et al. J Thromb Haemost. 2012;10(7):1342-1351.
- Szecsi PB, et al. Thromb Haemost. 2010;103(4):718-727.
- Curry N, et al. Crit Care Resusc. 2023;23(1):32-46.
- Winearls J, et al. Crit Care Resusc. 2023;23(1):32-46.
- Neerman-Arbez M, Casini A. Int J Mol Sci. 2018;19(1):192.
- Casini A, et al. J Thromb Haemost. 2017;15(5):876-888.
- Casini A, de Moerloose P. Haemophilia. 2020;26(1):25-32.
- Valiton V, et al. Haemophilia. 2019;25(5):747-754.
- Mackie I, et al. Int J Lab Hematol. 2024;46(1):20-32.
- Casini A, et al. J Thromb Haemost. 2018;16(9):1887-1890.
- Leal-Noval SR, Del Rincón JPM. Blood Transfus. 2025;23(1):75-78.
- Chandler WL, et al. Transfusion. 2010;50(12):2547-2552.
- Levy JH, Welsby I. The Hematologist. 2025;22(3):2025314.
- Hartmann J, et al. Res Pract Thromb Haemost. 2022;7(1):100031.
- Casini A, et al. Thromb Haemost. 2018;118(11):1867-1874.
- Rossaint R, et al. Crit Care. 2023;27(1):80.
- Kietaibl S, et al. Eur J Anaesthesiol. 2023;40(4):226-304.
- National Blood Authority. Patient blood management guideline for adults with critical bleeding. v1.7. Updated May 28, 2024. Accessed July 24, 2025. https://app.magicapp.org/#/guideline/Evqmmn
- Raphael J, et al. Anesth Analg. 2019;129(5):1209-1221.
- Curry NS, et al. Br J Haematol. 2018;182(6):789-806.
- Levy JH, et al. Transfusion. 2014;54(5):1389-1405.
- Holcomb JB, et al. J Trauma Acute Care Surg. 2013;75(1 suppl 1):S31-S39.
- Cushing MM, et al. Transfusion. 2020;60(suppl 3):S17-S23.
- CSL Behring receives FDA approval of RiaSTAP, first and only approved treatment of acute bleeding episodes in patients with congenital fibrinogen deficiency. BioSpace. January 20, 2009. Accessed February 25, 2025. www.biospace.com/csl-behring-receives-fda-approval-of-riastap-tm-first-and-only-approved-treatment-of-acute-bleeding-episodes-in-patients-with-congenital-fibrinogen
- Kreuz W, et al. Transfus Apher Sci. 2005;32(3):247-253.
- Rahe-Meyer N, et al. J Thorac Cardiovasc Surg. 2013;145(3 suppl):S178-S185.
- Cui Y, et al. Artif Organs. 2010;34(11):955-960.
- Schöchl H, et al. Crit Care. 2011;15(2):R83.
- CSL. Product pipeline. Accessed July 25, 2025. www.csl.com/research-and-development/product-pipeline
- Rahe-Meyer N, et al. Clin Appl Thromb Hemost. 2024;30:10760296241254106.
- Octopharma. FDA Approves fibryga for acquired fibrinogen deficiency, potentially ushering in a new standard of care (press release). September 8, 2024. Accessed February 25, 2025. www.octapharma.com/news/press-release/2024/fda-approves-fibryga-for-acquired-fibrinogen-deficiency
- Khayat CD, et al. Transfusion. 2022;62(9):1871-1881.
- Callum J, et al. JAMA. 2019;322(20):1966-1976.
- Deswal, P. Grifols gears up for FDA approval of fibrinogen replacement therapy. Clinical Trials Arena. February 14, 2024. Accessed February 25, 2025. www.clinicaltrialsarena.com/news/grifols-gears-up-for-fda-approval-of-fibrinogen-replacement-therapy/
- Roy A, et al. PB1217. Presented at: Research and Practice in Thrombosis and Haemostasis. Washington, DC: June 21-25, 2025.
- Trouillier H-H, et al. PB1219. Presented at: Research and Practice in Thrombosis and Haemostasis. Washington, DC: June 21-25, 2025.
- ClinicalTrials.gov. Phase 3 study of fibrinogen concentrate (CSL511) in subjects with pseudomyxoma peritonei undergoing cytoreductive surgery (NCT06617897), May 8, 2025. Accessed July 25, 2025. www.clinicaltrials.gov/study/NCT06617897
- Pastier C, et al. J Surg Oncol. 2024;130(6):1316-1325.
false
*You must be logged in to see results data.
- Fibrinogen has a role in facilitating of the following except:
- Platelet aggregation
- Thrombin cleavage
- Clot formation
- Wound healing
Correct answer: b.During the coagulation cascade, thrombin cleaves fibrinogen, releasing fibrinopeptides. This process allows fibrin monomers to self-polymerize and form a stable clot. Fibrinogen facilitates platelet aggregation, modulates inflammation, and contributes to wound healing. - In the clinical care setting, fibrinogen levels below __ g/dL are an independent risk factor for severe hemorrhage and transfusion requirements in cases of trauma, surgery, and gynecologic and obstetric complications.
- 1.2
- 1.6
- 1.8
- 2
Correct answer: d.A fibrinogen level lower than the normal level of 200 mg/dL (2 g/dL) is recognized as an independent risk factor for severe hemorrhage and the need for transfusions. - Which of the following statements best describes acquired fibrinogen deficiencies?
- They are rare, representing an estimated 8% of the rare bleeding disorders.
- They are caused by mutations in the FGA, FGB, and FGG genes.
- They can arise from external factors that disrupt fibrinogen synthesis, function, or consumption of fibrinogen.
- Patients often present in early childhood and may experience spontaneous bleeding episodes.
Correct answer: c.Acquired fibrinogen deficiencies can arise from external factors that either disrupt the synthesis or function of fibrinogen, or lead to its consumption. The 8% prevalence applies to congenital, not acquired, deficiencies. Genetic mutations and early childhood onset are features of congenital, not acquired, deficiency. - True or false: The Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis developed a classification system for congenital fibrinogen deficiencies in 2018 based on symptom presentation and severity and fibrinogen levels to aid in the diagnosis and differentiation of these cases.
- True
- False
Correct answer: a.The Scientific and Standardization Committee developed a classification system for congenital fibrinogen deficiencies in 2018. - Which of the following is the most used test for evaluating fibrinogen function?
- Clauss assay
- Viscoelastic testing (VET)
- Thrombin clotting time (TT)
- Fibrinogen antigen testing
Correct answer: a.Newer guidelines are starting to recommend the use of newer, more reliable VETs when available, but the Clauss assay remains the mainstay. TT is used as an initial screen and has low specificity—not the main method. Fibrinogen antigen testing measures total protein, not functional activity. - Genotyping may be considered as part of the stepwise diagnosis workup for congenital fibrinogen deficiency for all the following reasons except:
- Genotyping can be used to screen family members.
- Genotyping can be used to differentiate among subtypes.
- Genotyping can be used to confirm a diagnosis of congenital fibrinogen deficiency.
- Genotyping can be used to evaluate the function of fibrinogen in an individual with congenital fibrinogen deficiency.
Correct answer: d.Genotyping should be considered to help confirm diagnosis and differentiate between subtypes, facilitate screening of family members. It does not assess fibrinogen functionality. - Approximately how many mL of cryoprecipitate are needed to deliver between 200 and 250 mg of fibrinogen to a patient in hemorrhagic crisis?
- 1 to 5
- 5 to 15
- 10 to 20
- 20 to 25
Correct answer: c.Each unit of cryoprecipitate, which typically ranges from 10 to 20 mL, contains approximately 200 to 250 mg of fibrinogen. - Which of the following therapies, if approved, will become the second fibrinogen concentrate (FC) indicated for acquired fibrinogen deficiency in the United States?
- Fresh frozen plasma (FFP)
- RiaSTAP
- CSL511
- BT524
Correct answer: d.If approved, BT524 will become the second FC specifically indicated for acquired fibrinogen deficiencies in the United States. FFP is not an FC, RiaSTAP is FDA-approved, and CSL511 is not yet at this stage of development. - A 16-year-old girl with congenital hypofibrinogenemia (diagnosed by prior bleeding and low Clauss assay, most recently 70 mg/dL) and mild chronic kidney disease from a congenital solitary kidney, is scheduled for laparoscopic appendectomy. The standard protocol calls for cryoprecipitate, but none is available on short notice. The nephrology team is also concerned about the risk for fluid overload. What should they do?
- Administer RiaSTAP 70 mg/kg
- Administer fibryga 70-75 mg/kg
- Either A or B
- None of the above
Correct answer: c.Both RiaSTAP 70 mg/kg and fibryga 70 to 75 mg/kg are FCs recommended for the treatment of congenital hypofibrinogenemia in surgical settings, especially when rapid, precise, and low-volume fibrinogen replacement is needed. Using either product at evidence-based doses ensures effective hemostasis and helps prevent complications like fluid overload in patients with kidney disease, making them interchangeable and preferable when cryoprecipitate is unavailable.
false