1.0 ACPE contact hour
Faculty
Siamak Daneshmand, MD
Professor of Urology and Medicine (Oncology), Clinical Scholar
Director of Urologic Oncology
Director of Clinical Research
Urologic Oncology Fellowship Director
USC/Norris Comprehensive Cancer Center
Los Angeles, CA
Fady Ghali, MD
Assistant Professor
Yale Cancer Center
Department of Urology
Yale School of Medicine
New Haven, CT
Goal
The goal of this program is to improve clinical decision-making and patient-centered care in non–muscle-invasive bladder cancer (NMIBC) by educating health care professionals (HCPs) on the role of novel intravesical gene therapies, evidence-based treatment pathways, and multidisciplinary strategies in addressing the needs of patients with BCG-unresponsive disease.
Intended Audience
This educational initiative is designed for US-based urologists, oncologists, hospital pharmacists, Veterans Health Administration pharmacists, and other HCPs involved in the care of patients with bladder cancer.
Educational Objectives
At the completion of the activity, participants will be better able to:
- Review the clinical burden and unmet needs associated with Bacillus Calmette-Guérin (BCG)-unresponsive NMIBC.
- Discuss the current treatment landscape in BCG-unresponsive NIMBC.
- Evaluate the mechanism of action, safety, efficacy, and clinical use criteria for an approved intravesical gene therapy in NMIBC.
- Outline how to incorporate patient-centered communication, shared decision-making, and health system navigation strategies to improve outcomes for patients with NMIBC.
Physician Accreditation Statement

Physician Credit Designation
Global designates this enduring activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Pharmacist Accreditation Statement

Pharmacist Credit Designation
Global designates this continuing education activity for 1.0 contact hour (0.1 CEU) of the ACPE (Universal Activity Number: 0530-9999-25-029-H01-P). This is a knowledge-based activity.
Instructions for Obtaining Credit
To receive credit, participants must participate in the activity and complete and pass the post-test at cmezone.com/cu252p with a minimum score of 70%. Additionally, participants will need to complete the evaluation. CME certificates will be sent via email to those successfully completing the activity.
Disclosures of Relevant Financial Relationships
Global adheres to the policies and guidelines, including the Standards for Integrity and Independence in Accredited CE, set forth to providers by the ACCME and all other professional organizations, as applicable, stating those activities where continuing education credits are awarded must be balanced, independent, objective, and scientifically rigorous. All persons in a position to control the content of an accredited continuing education program provided by Global are required to disclose all financial relationships with any ineligible company within the past 24 months to Global. All financial relationships reported are identified as relevant and mitigated by Global in accordance with the Standards for Integrity and Independence in Accredited CE in advance of delivery of the activity to learners. The content of this activity was vetted by Global to assure objectivity and that the activity is free of commercial bias. All relevant financial relationships have been mitigated.
The faculty have the following relevant financial relationships with ineligible companies:
- Siamak Daneshmand, MD: Consulting fees (eg, advisory boards): AstraZeneca, BMS, CG Oncology, Engene, Ferring Pharmaceuticals, Immunitybio, Johnson and Johnson, Pacific Edge, Pfizer, Photocure, Urogen; contracted research (principal investigators must provide information, even if received by the institution): Photocure; other (Restricted Class B Incentive Unit Agreement): Taris
- Fady Ghali, MD: Consulting fees (eg, advisory board): Urogen (ended 12/2024); contracted research (principal investigators must provide information, even if received by the institution): Ferring Pharmaceuticals; honoraria: Clinical Care Options (ended 12/2024)
The planners and managers have the following relevant financial relationships with ineligible companies:
- The planners and managers at Global have no relevant financial relationships to disclose.
- The planners and managers at ACE have no relevant financial relationships to disclose.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents not indicated by the FDA. Global and ACE do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of any organization associated with this activity. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed in this activity should not be used by clinicians without evaluation of patient conditions and possible contraindications on dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
System Requirements
For technical support, please contact ACE at (212) 957-5300, ext 280 or cmezonesupport@appliedcme.com.
Global Contact Information
For information about the accreditation of this program, please contact Global at (303) 395-1782 or cme@globaleducationgroup.com.
Fee Information and Refund/Cancellation Policy
There is no fee for this educational activity.
This activity is jointly provided by Global Education Group and Applied Clinical Education.

This activity is supported by an educational grant from Ferring Pharmaceuticals.
This activity is distributed by Pharmacy Practice News, Clinical Oncology News, and CMEZone.com.

false
*You must be logged in to see results data.
The management of non–muscle-invasive bladder cancer (NMIBC) can represent a significant clinical challenge. High-risk patients and those unresponsive to first-line standard-of-care (SOC) treatment face a substantial risk for disease progression, recurrence, and bladder loss, which can adversely affect their quality of life (QoL) and survival. Recent years have witnessed a shift in the management of these cases, with the emergence of new bladder-sparing options. Understanding the epidemiology, evolving diagnostic and risk stratification tools, mechanisms of therapeutic resistance, and positioning of new treatments is central to improving patient outcomes in this complex disease state.
Bladder cancer poses a significant public health challenge in the United States. In 2025, it is expected to be the 6th most commonly diagnosed cancer overall, with about 84,870 new cases, and the 10th leading cause of cancer death, responsible for an estimated 17,420 deaths.1 Incidence is notably higher in men, who are diagnosed nearly 4 times as often as women, making bladder cancer the 4th most common cancer in men and the 11th in women.1-3 More than 593,000 individuals currently live with bladder cancer in the United States.4
The disease predominantly affects older adults, with a median age at diagnosis of 73 years, and more than 90% of cases occurring in individuals aged 55 or older. Incidence peaks between ages 70 and 80, reflecting a strong age-dependent risk.2 During the past decade, both incidence and mortality have declined by approximately 1% per year, likely owing to reductions in cigarette smoking, advances in early detection, and modern treatment approaches.1,2 Despite these improvements, outcomes remain closely tied to disease stage at diagnosis. The overall 5-year survival rate for bladder cancer is about 78%. However, this decreases to 40% for patients with lymph node involvement and 9% to 29% for those with distant metastasis.1 These statistics underscore the critical need for prevention and timely intervention.
Geographic trends demonstrate a higher incidence of bladder cancer in industrialized and urban areas, likely influenced by occupational exposure to carcinogenic chemicals.1 Racial and ethnic disparities persist: White men have the highest incidence, whereas Black and Hispanic patients often present at more advanced disease stages and experience worse outcomes.2,5,6 Black patients are also more likely to be diagnosed with variant histology, which is generally considered a high-risk feature that limits treatment options.5,6
Histologically, approximately 90% of bladder cancers diagnosed in the United States are urothelial (transitional cell) carcinomas, originating in the transitional epithelium. The remainder are squamous cell carcinomas (2%-7%) and adenocarcinomas (2%).3,7 Tumors are further classified morphologically as either sessile (flat) or papillary, and by grade as low- or high-grade.3 From a clinical perspective, distinguishing between muscle-invasive and non–muscle-invasive disease is crucial for determining management strategy. At diagnosis, approximately 75% of cases are non–muscle-invasive.8
NMIBC remains uniquely challenging due to its high rates of recurrence and progression. One-year recurrence rates range from 15% to 61%, and by 5 years, as many as 78% of patients experience a recurrence. This makes NMIBC the most recurrent malignancy among common solid tumors.9 These high recurrence rates generate a substantial economic burden, as the costs of treating NMIBC, including surveillance and repeated interventions, make up the majority of bladder cancer-related health care expenditures.10 For patients who do not respond to initial treatment and require radical cystectomy, costs increase significantly.
The single most significant modifiable risk factor for bladder cancer is tobacco use, accounting for nearly 50% of all new cases. Carcinogenic chemicals from smoking are filtered and concentrated in the urine, prolonging exposure to the bladder lining and increasing mutational risk.1,3 Other important risk factors include male sex, age older than 55 years, chronic bladder inflammation (from infections or long-term catheter use), prior pelvic radiation, and prior exposure to chemotherapeutic agents such as cyclophosphamide.1,3
Occupational exposure to aromatic amines—chemicals used in dyes, rubber, leather, petroleum, and various manufacturing industries—also represents a prominent risk factor. Chronic exposure to these compounds is strongly associated with bladder cancer, especially among workers in high-risk occupations.1,3
Military service also confers elevated risk. Bladder cancer is the fourth most common cancer among US veterans, with about 3200 new cases diagnosed annually. Veterans are 2 to 3 times more likely than the general public to be diagnosed with bladder cancer, a disparity attributed to toxic exposures (eg, Agent Orange, dioxins, depleted uranium), the demographic predominance of men, higher rates of smoking, and occupational hazards encountered during service.11-13 Diagnosis may be delayed in veterans due to subtle symptoms that may be downplayed, overlooked, or misattributed to other issues such as post-traumatic stress disorder (PTSD), the presence of complex comorbidities, and a reduced willingness to seek medical attention or cope with a cancer diagnosis.12,13
NMIBC presents with a broad spectrum of symptoms. The most common presenting feature is hematuria, either gross or microscopic, seen in up to 85% of patients. Hematuria is typically painless and may be intermittent, which can contribute to diagnostic delays. Irritative voiding symptoms such as urinary frequency, urgency, and dysuria are less common but may signal carcinoma in situ (CIS), and are frequently mistaken for urinary tract infection (UTI) or benign prostatic hyperplasia, especially in older adults.14,15 A significant minority of patients remain asymptomatic. In some cases, NMIBC is detected during evaluation for persistent microscopic hematuria or recurrent infection or is incidentally identified on imaging for unrelated indications.16
The diagnosis of NMIBC is complicated by the absence of validated noninvasive screening tools and limitations in the sensitivity of available adjunct tests (Table 1).15-22 Thus, many cases are diagnosed at advanced stages.23 Cystoscopy remains the gold standard, offering high sensitivity (approximately 85%-90%) and specificity (87%-100%) for visible urothelial lesions. It is routinely performed in the office setting and permits direct visualization, biopsy, and resection when needed. Flat lesions, such as CIS, may be missed, underscoring the importance of both operator experience and the use of ancillary testing.15,16
| Table 1. Diagnostic Modalities in Bladder Cancer.15-22 | ||||
| Test | Sensitivity, % | Specificity, % | Clinical role | Comments |
|---|---|---|---|---|
| Cystoscopy | ~85-90 | ~87-100 | First-line tool for detecting visible lesions | Gold standard; limited in detecting flat lesions like CIS15 |
| Blue light cystoscopy | ~93-96 | ~46 | Adjunct to standard cystoscopy | Detects additional lesions, particularly CIS; improves detection, lowers recurrence and progression; requires HAL agent and special equipment17,18 |
| Urine cytology | ~20-40 overall; up to 80-90 for CIS | ~90-98 | Adjunctive tool, especially for high-grade disease | Poor sensitivity for low-grade tumors; widely available19 |
| FISH (eg, UroVysion) | ~60-80 | ~65-80 | Detects chromosomal abnormalities in voided urine | Useful for ambiguous cytology; higher cost20 |
| NMP22 (nuclear matrix protein) | ~55-70 | ~60-85 | Urine-based tumor marker | Higher false positives in inflammation or infection21 |
| Bladder tumor antigen (BTA-TRAK/Stat) | ~50-70 | ~60-80 | Antibody-based ELISA | Poor specificity in benign urologic conditions22 |
| CT urography | ~80-85 (for upper tract) | ~90 | Excludes upper tract urothelial carcinoma | Less helpful for primary NMIBC; exposes to radiation and contrast16 |
| CIS, carcinoma in situ; CT, computed tomography; ELISA, enzyme-linked immunosorbent assay, FISH, fluorescence in situ hybridization; HAL, hexaminolevulinate; NIMBC, non–muscle-invasive bladder cancer. | ||||
Blue light cystoscopy is an advanced diagnostic technique that uses hexaminolevulinate, a photosensitizing agent, which causes cancerous cells to fluoresce under blue light, making them more readily distinguishable from normal tissue.17 When illuminated by blue light (380-450 nm), cancerous cells emit red fluorescence, whereas normal cells appear blue.17 This technique can significantly increase sensitivity for detection, particularly of flat CIS or papillary tumors, and has been shown to improve clinical outcomes by reducing recurrence and progression rates compared with conventional (white light) cystoscopy.24
Urine cytology, which is widely accessible, has high specificity and is most sensitive for detecting high-grade lesions or CIS (60%-70%) but demonstrates poor sensitivity for low-grade tumors. Thus, it functions primarily as an adjunct tool for high-risk patients and during surveillance.15,19
Molecular assays, such as fluorescence in situ hybridization (UroVysion), NMP22, and the BTA-TRAK or BTA-Stat test, provide moderate sensitivity (usually 50%-80%) and are not routinely used. The utility of these assays is limited by higher cost, lower specificity (with false positives frequently resulting from benign conditions such as inflammation or UTI), and inconsistent insurance reimbursement.20-22 Advanced molecular diagnostics are less accessible in rural or resource-limited environments.22 Computed tomography (CT) urography is primarily used to exclude upper tract involvement in patients with hematuria but plays a limited role in primary NMIBC diagnosis.16
The most typical scenario is diagnosis of stage Ta low-grade NMIBC following evaluation for painless hematuria. However, 40% to 50% of new cases already present with high-grade disease—most notably those with CIS or persistent/recurrent symptoms—and these patients require prompt risk stratification and aggressive management.14-16,19,25
Transurethral resection of bladder tumor (TURBT) is the initial and definitive diagnostic and therapeutic procedure for patients with suspected bladder neoplasms. It remains the gold standard for establishing a pathologic diagnosis, assessing tumor stage and grade, and providing the majority of patients with their first therapeutic intervention. TURBT is typically performed under regional or general anesthesia using a resectoscope passed through the urethra. The main objective is complete gross tumor resection and representative sampling of the underlying and adjacent bladder wall for staging and grading.15
The completeness of initial TURBT is crucial. Removal of all visible tumor tissue, along with adequate sampling of underlying detrusor muscle, is necessary to distinguish non–muscle-invasive from muscle-invasive disease and to avoid under-staging. Under-sampling or incomplete resection may result in missed identification of T1 or T2 disease and thus, suboptimal treatment stratification. For high-risk lesions—particularly T1 tumors, high-grade disease, or specimens lacking muscle tissue—a repeat TURBT is recommended within 2 to 6 weeks to ensure accurate staging and reduce the risk for progression.14-16
In addition to establishing diagnosis and primary management, TURBT enables the identification of CIS, which can appear as a flat erythematous lesion or in regions distant from the primary tumor. Advanced imaging techniques, such as narrow-band imaging, may improve the detection of CIS and completeness of initial resection, especially in high-risk patients. However, this technology may have limited availability, particularly in community or under-resourced settings.14-16
Complications from TURBT are relatively infrequent but can include bleeding, bladder perforation, and infection. Rare but important risks include obturator reflex with resultant bladder wall injury and, in patients with deep resection near the ureteral orifices, risk for ureteric injury or stricture.
The diagnosis and risk stratification of NMIBC relies on a combination of tumor stage—based on the American Joint Committee on Cancer (AJCC)/TNM system—and tumor grade, typically using the World Health Organization (WHO) 2004/2016 grading system.15,26
- Stage Ta tumors are noninvasive papillary lesions confined to the urothelium.
- Stage T1 tumors invade the lamina propria but not the muscularis propria.
- CIS is, by definition, a flat, high-grade, noninvasive lesion limited to the urothelium, frequently multifocal, and prone to rapid progression if not promptly managed.15,16
Tumor grade is a major prognostic factor and determinant of therapy. The 2004/2016 WHO grading system designates tumors as low-grade or high-grade, superseding the older 3-tiered system (G1-G3). Low-grade tumors display fewer mitotic features and a more orderly architecture, whereas high-grade tumors are pleomorphic and more aggressive.15,26
Multiple tumors, large size (>3 cm), multifocality, frequent recurrences, concomitant CIS, deep lamina propria invasion (T1), and lympho-vascular invasion are all features associated with higher risk for progression and recurrence. The presence of subtype histology or divergent differentiation (eg, micropapillary, plasmacytoid) also signals a higher risk and may warrant aggressive management.14-16
Careful annotation of tumor number, size, site, and the presence or absence of muscle in the resected specimen should be included in the pathology report to optimally inform treatment planning.15,16,25
After complete TURBT and pathologic confirmation, patients with NMIBC must be stratified into risk categories to guide further management. Contemporary guidelines from the American Urological Association (AUA), the European Association of Urology (EAU), and the National Comprehensive Cancer Network (NCCN) recommend classifying patients as low-, intermediate-, or high-risk for recurrence and progression based on an integrated assessment of stage, grade, tumor size, focality, presence of CIS, and other adverse features.14-16,27
Low-risk NMIBC refers to solitary, low-grade Ta papillary tumors that are 3 cm or smaller, without evidence of CIS, and no prior recurrences. Patients with low-risk NMIBC have an excellent prognosis, with management strategies designed to maximize effectiveness while minimizing overtreatment.15 Complete TURBT may be sufficient in many cases; however, guidelines recommend a single immediate postoperative intravesical chemotherapy instillation within 24 hours of TURBT.16,28 This practice reduces the risk for recurrence by approximately 35%, based on high-level evidence from multiple randomized trials and meta-analyses.28
Both gemcitabine and mitomycin C are cytotoxic agents originally approved for intravenous administration in the treatment of various solid tumors; their intravesical use in NMIBC is off-label, but it is endorsed by the NCCN and EAU guidelines. Notably, intravesical administration enables direct, localized drug activity within the bladder while minimizing systemic absorption and associated toxicity.15,16 Gemcitabine was first approved by the FDA in 1996, and has since become widely used in bladder cancer; mitomycin C, FDA-approved in 1974, has been used off-label for intravesical therapy since the late 1980s and has become an internationally accepted SOC in this context.15,28
After TURBT and a single perioperative dose of chemotherapy, no further intravesical therapy is required in most low-risk cases. The risk for recurrence is low, and the expected risk for disease progression is essentially zero. Ongoing management focuses on careful surveillance involving periodic cystoscopy to detect new tumors at an early, easily curable stage.15,16
Intermediate-risk NMIBC encompasses patients with multiple and/or large low-grade Ta lesions, early recurrences, or some but not all high-risk features. This includes recurrent or multifocal low-grade Ta tumors, as well as solitary low-grade Ta lesions larger than 3 cm.15 The primary goal for this cohort is to reduce the risk for recurrence, prolong disease-free intervals, and minimize overtreatment.
The backbone of management for intermediate-risk NMIBC is complete TURBT followed by a 6-week course of an intravesical agent such as gemcitabine or mitomycin C.15,16 Maintenance chemotherapy may be considered for up to 1 year in patients with higher recurrence risk or significant tumor burden; this approach has been shown to extend recurrence-free survival and diminish the need for repeat interventions in carefully selected populations.15,29
UGN-102
A notable new option for intermediate-risk disease is UGN-102, a reverse thermal gel formulation of mitomycin C approved by the FDA in August 2025 and intended for patients with recurrent low-grade intermediate-risk NMIBC who are poor candidates for surgical resection.30-32 The drug is administered via a urinary catheter once weekly for 5 consecutive weeks. Unlike traditional aqueous mitomycin C, which is difficult to retain for more than 1 to 2 hours, UGN-102 is instilled as a liquid and gels at body temperature, remaining in the bladder for 4 to 6 hours to promote consistent mucosal exposure.30,32
Approval was based primarily on the single-arm, open-label phase 3 ENVISION trial, which included patients with recurrent low-grade intermediate-risk NMIBC. In this study, 78% of patients achieved a complete response (CR) at 3 months, and 82.3% maintained that response for at least 12 months.30,31 Adverse events (AEs) were typically mild and included dysuria (25%), urinary urgency (20%), hematuria (15%), and fatigue (12%).
In contrast, the phase 3 ATLAS trial was a randomized, controlled study comparing UGN-102 as monotherapy to standard TURBT in patients with either newly diagnosed or recurrent low-grade intermediate-risk NMIBC. UGN-102 demonstrated a 3-month CR rate of 63.6%, similar to that seen with TURBT (64.8%); at 12 months post-treatment, 79.7% and 67.7% of patients remained disease-free after UGN-102 and TURBT, respectively. The hazard ratio for duration of response (DoR; 0.46) favored UGN-102, indicating a lower risk for recurrence compared with surgery alone. Dysuria was slightly more common in ATLAS (30.4%) vs ENVISION (22.5%).33
High-risk NMIBC is defined by the presence of any of the following features: high-grade Ta, T1, CIS, multiple and large tumors, variant histology, or lympho-vascular invasion.15,16 These cases require aggressive management, typically with induction and maintenance Bacillus Calmette-Guérin (BCG) therapy, rigorous surveillance including upper-tract imaging, and prompt re-resection if high-risk features are confirmed.14-16 Therapies for high-risk disease are summarized in the next section.
BCG is the foundational, guideline-endorsed therapy for patients with high-risk NMIBC, including with CIS, high-grade Ta, and T1 tumors after complete TURBT.34 BCG consists of a live, attenuated strain of Mycobacterium bovis, originally developed as a tuberculosis vaccine and approved for bladder cancer treatment in 1990 based on its ability to stimulate a robust, localized immune response against residual and microinvasive urothelial cancer cells.34 Following instillation directly into the bladder via catheter, BCG attaches to urothelial cells, is internalized, and triggers a cascade of immunologic processes. The mechanism involves activation of resident macrophages and dendritic cells, leading to the recruitment of cytotoxic CD4+ and CD8+ T cells. This interferon (IFN)- and cytokine-enriched environment, particularly involving T-helper 1 cytokines like interleukin-2 (IL-2) and IFN-γ, results in the direct lysis and clearance of malignant cells.35,36
Standard BCG induction consists of once-weekly intravesical instillations for 6 consecutive weeks, delivered after grossly complete TURBT. Maintenance therapy is then recommended at 3, 6, and 12 months, and may be extended up to 3 years in patients with continued high-risk features or CIS, as validated in randomized trials and major guideline recommendations.15,16,34 BCG provides high efficacy for NMIBC: CR rates in CIS approach 70%, and long-term randomized and pooled data confirm significant reductions in both recurrence and progression for patients receiving both induction and maintenance therapy.36 These results have anchored BCG as the international SOC for more than 3 decades.
Despite its history of success, BCG is associated with several drawbacks (Table 2), including a spectrum of AEs.37-39 The most common AEs are localized and include dysuria, increased urinary urgency or frequency, mild fever, and self-limited hematuria. Most are mild to moderate, but rare and clinically significant complications—including systemic BCG infection (BCG sepsis), fever exceeding 38.5∞C, or disseminated granulomatous disease—can occur, especially if mucosal integrity is compromised or the bladder wall is traumatized during catheterization.34 Important contraindications include immunosuppression (related to HIV/AIDS, leukemia, lymphoma, or chronic corticosteroid use); active UTI; febrile illness; gross hematuria; or recent bladder instrumentation, biopsy, or surgery within the past 7 to 14 days.34
| Table 2. Drawbacks to BCG in Non–Muscle-Invasive Bladder Cancer37-39 | |
| Drawback | Description/Examples |
|---|---|
| High rate of AEs | >70% of patients experience some side effects, including irritative urinary symptoms, cystitis, hematuria, and fever |
| Serious AEs leading to discontinuation | Approximately 8%-10% of patients must stop BCG due to toxicity, including severe bladder complications or sepsis |
| Reduced tolerance with full dose or maintenance | Full-dose and longer maintenance increase side effects vs lower-dose or combination therapy |
| Incomplete treatment adherence | Toxicity and AEs result in low completion rates; only 16% in some studies complete full induction plus maintenance therapy |
| Risk for failure (non-responsiveness) | ≤40% of patients may not respond, requiring alternative therapy or radical cystectomy, especially for high-risk disease |
| Systemic complications | Rare but serious risks include BCG sepsis, prostatitis, joint pain, rash, malaise, and incontinence |
| Exacerbated by comorbidities or infections | Urinary tract infections or bladder trauma can worsen or contraindicate BCG use |
| Global shortages | Supply issues have interrupted care, increasing the need for alternatives |
| AE, adverse event; BCG, Bacillus Calmette-Guérin. | |
A current challenge in clinical practice is the ongoing shortage of BCG, which has disrupted treatment protocols worldwide. Clinicians have often been forced to use reduced dosing regimens (ie, one-third of the standard dose) or to ration full-dose BCG, prioritizing patients with the highest-risk disease.40-42 These shortages affect the availability of both induction and maintenance courses, occasionally necessitating alternative intravesical therapies or enrollment in clinical trials.
Furthermore, up to 40% of patients will not respond to BCG or will experience disease recurrence (BCG failure), even after completing an adequate course of treatment.14,43,44 These cases present the greatest risk for progression and have represented a major clinical dilemma. However, the recent development of alternative and gene therapies offers new hope for these patients.
BCG-UNRESPONSIVE NMIBC
Precisely defining BCG unresponsiveness is crucial for clinical decision-making, regulatory clarity, and trial eligibility. Consensus definitions from the FDA, AUA, and EAU, as well as current NCCN guidelines, define BCG-unresponsive NMIBC as shown in Table 3.14-16,45
| Table 3. Defining BCG-Unresponsive NMIBC4 | ||
| Subcategory | Definition | Key Features |
|---|---|---|
| BCG-refractory | Persistent high-grade disease at 6 mo (or at 3 mo with worsening disease) despite adequate BCG | Indicates primary failure; unlikely to respond to further BCG |
| BCG-relapsing (early) | Recurrence of high-grade tumor within 6-12 mo after a CR to adequate BCG | High risk for progression; often treated as unresponsive |
| BCG intolerance | Inability to complete BCG due to severe adverse events | May be considered unresponsive if recurrence occurs |
| BCG-resistant (less commonly used term) | High-grade recurrence after initial response but after 12 mo | May still be eligible for retreatment depending on timing |
| BCG, Bacillus Calmette-Guérin; CR, complete response. | ||
Adequate BCG is characterized as the receipt of at least 5 of 6 induction doses and at least 2 of 3 maintenance doses (or a second induction course). If patients have not received this full regimen, they are often labeled “BCG-exposed” and may still be eligible for re-induction rather than being considered truly unresponsive.14,16
A designation of BCG-unresponsive carries important regulatory and clinical implications, as it defines eligibility for subsequently approved or experimental therapies. Patients with high-risk NMIBC, especially those with CIS, T1, or persistent/recurrent high-grade lesions after adequate BCG, face a substantial risk for disease progression to muscle-invasive disease and metastasis. In this group, timely identification and a frank discussion of treatment options—including radical cystectomy as the gold standard therapy, and newer FDA-approved and guideline-supported bladder-sparing approaches (Figure 1)46—is paramount for optimizing survival while supporting patient preferences for bladder preservation.16
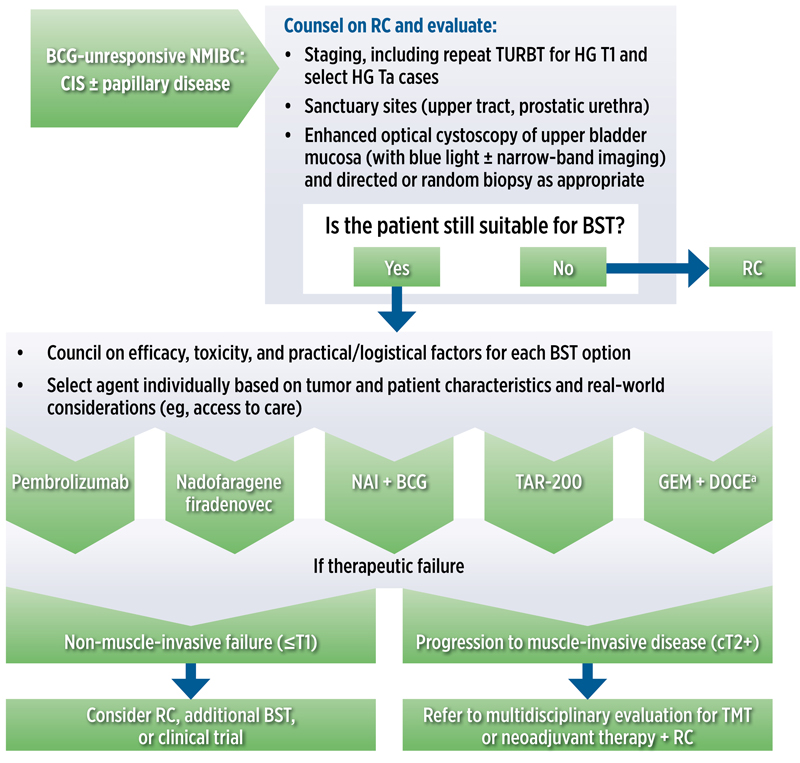 |
| Figure 1. Algorithm for BST in patients with BCG-unresponsive NMIBC who are ineligible for or refuse RC. a Not specifically approved for this use but may be considered based on available evidence. BCG, Bacillus Calmette-Guérin; BST, bladder-sparing treatment; CIS, carcinoma in situ; GEM + DOCE, gemcitabine + docetaxel; HG, high grade; NAI, nogapendekin alfa inbakicept; NMIBC, non–muscle-invasive bladder cancer; RC, radical cystectomy; TAR-200, gemcitabine intravesical system; TUR, transurethral resection of bladder tumor; TMT, trimodal therapy. Adapted from reference 46. |
For patients who do not respond to BCG therapy, the next traditional treatment option is radical cystectomy. This procedure entails complete surgical removal of the bladder along with adjacent organs (the prostate and seminal vesicles in men; uterus and anterior vaginal wall in women); pelvic lymphadenectomy; and urinary diversion via an ileal conduit, continent cutaneous reservoir, or orthotopic neobladder.15 When performed early for high-risk NMIBC, radical cystectomy is associated with greater than 90% cancer-specific survival at 5 years.14
Nevertheless, radical cystectomy is a major surgery with a high risk for complications. Data from the United States Radical Cystectomy Consortium reflect a 90-day overall complication rate of 64%, with 13% classified as major (Clavien grade III-V) and 51% as minor (Clavien I-II). Other studies report complication rates between 30% and 50%, especially in older patients, those with comorbidities, and at lower-volume centers.47-49 Complications include bowel obstruction, anastomotic leak, UTI, sepsis, and venous thromboembolism.43,50-52
QoL may be profoundly affected. Patients must adjust to altered body image, daily routines centered on stoma or pouch/appliance care (for ileal conduits or continent reservoirs), and learn new techniques for continence control or intermittent self-catheterization.53 Long-term challenges include stomal complications, incontinence, and “right-time” catheterization. Sexual dysfunction is common. Up to 80% of men may experience erectile dysfunction due to nerve damage, and loss of ejaculation is widespread. Women may face vaginal shortening, dyspareunia, and surgical menopause.54,55 Psychosocial burdens, such as anxiety, depression, and social withdrawal related to appliance concerns or body image, are well documented.56
Despite these challenges, QoL after cystectomy may compare favorably to bladder-sparing therapies, which can help patients facing difficult treatment decisions.57
Recently, a shift toward bladder-sparing, non-surgical treatments has transformed the management landscape for BCG-unresponsive NMIBC—a setting where, until very recently, radical cystectomy or experimental regimens were the only alternatives. Table 4 provides summaries of key bladder-sparing treatment options for BCG-unresponsive high-risk NMIBC.58-70
| Table 4. Key Bladder-Sparing Therapies for BCG-Unresponsive High-Risk NMIBC58-70 | |||||
| Drug | Pembrolizumab | Nadofaragene firadenovec | Nogapendekin alfa inbakicept + BCG | Gemcitabine intravesical system | Gemcitabine + docetaxel |
|---|---|---|---|---|---|
| Mechanism of action | Anti–PD-1 monoclonal antibody—restores immune activity by blocking PD-1/PD-L1 axis | Nonreplicating adenoviral vector delivers IFN-α2b gene to urothelial cells, producing local interferon and immune activation | IL-15 receptor superagonist—stimulates NK cells and CD8? T cells, synergizes with BCG | Inhibition of DNA synthesis; sustained-release (device) | Gemcitabine inhibits DNA synthesis; docetaxel induces mitotic arrest and apoptosis |
| Indication | BCG-unresponsive high-risk NMIBC with CIS ± papillary tumors when cystectomy refused/ineligible58 | BCG-unresponsive high-risk NMIBC with CIS ± papillary tumors60 | BCG-unresponsive with CIS ± papillary tumors, given with BCG63 | BCG-unresponsive NMIBC with CIS ± papillary tumors65 | Used off-label for BCG-unresponsive NMIBC; recurrent intermediate-/high-risk disease16,27 |
| Administration and dosing | 200 mg IV every 3 wk for ≤24 mo | 75 mL instilled every 3 mo | Weekly ×6 (induction), then weekly for 3 wk at months 4, 7, 10, 13, and 19 (maintenance), with BCG | 225 mg; device inserted into bladder; replaced every 3 wk ×6 cycles (induction) then every 12 wk (maintenance) for ≤24 mo | 1 g gemcitabine, followed by 37.5 mg docetaxel weekly ×6 (induction), then monthly for ≤24 mo (maintenance) |
| Key trial(s) | KEYNOTE-057 (phase 2) | NCT02773849 (phase 3) | QUILT 3.032 (phase 2/3) | SunRISe-1 (phase 2b) | NCT02202772, NCT01619363, multi-institutional studies |
| Key efficacy outcomes | 41% CR at 3 mo; median DoR 16.2 mo; 46% maintained at 12 mo59 | 51% CR at 3 mo; 45% maintained at 12 mo; median DoR 9.7 mo; 24-mo RFS 17%; 75% cystectomy-free at 12 mo61 | 71% CR; 45% at 12 mo; 91% cystectomy-free at 24 mo in CIS cohort64 | 82% CR; 46% maintained at 12 mo; median DoR 25.8 mo66 | 48%-54% RFS at 2 y; 75% bladder preservation at 5 y; comparable/superior to BCG salvage in multicenter 2025 study67-70 |
| Common AEs | Fatigue, pruritus, diarrhea, immune-related AEs (colitis, pneumonitis, endocrinopathies)58,59 | Fatigue (23%), bladder spasm (18%), urgency (17%), hematuria (12%); nearly all grade 1-261,62 | Dysuria (36%), fatigue (25%), frequency (20%), nocturia (18%); some grade 3 hematuria, UTI, immune events, rare grade 564 | Pollakiuria (44%), dysuria (40%), micturition urgency (25%), UTI (21%); grade ≥3 AEs, 12.%; serious AEs, 5.9%66 | Dysuria, frequency, mild local cystitis67,70 |
| FDA approval | 2020 | 2022 | 2024 | 2025 | Not FDA-approved for this use but guideline-supported16,27 |
| AE, adverse event; BCG, Bacillus Calmette-Guérin; CIS, carcinoma in situ; CR, complete response; DoR, duration of response; IFN, interferon; IL, interleukin; IV, intravenous; NK, natural killer; NMIBC, non–muscle-invasive bladder cancer; PD, programmed death; RFS, recurrence-free survival; UTI, urinary tract infection. | |||||
Pembrolizumab is an immune checkpoint inhibitor targeting the programmed death (PD)-1 protein that promotes immune-mediated clearance of tumor cells. The FDA approved pembrolizumab in 2020 for patients with BCG-unresponsive, high-risk NMIBC with CIS, who are either ineligible for or decline cystectomy.58 Its use remains off-label for papillary disease or for use in combination regimens outside this indication.
In the pivotal KEYNOTE-057 phase 2 trial, single-agent pembrolizumab achieved a 40.6% CR rate at 3 months, with a median DoR of 16.2 months.59 Among responders, only about one-third maintained response at 18 months and 23% at 24 months.
Treatment is administered intravenously at 200 mg every 3 weeks, for up to 2 years. Because pembrolizumab is administered systemically, it necessitates collaboration between urology and medical oncology providers and carries risks for immune-related AEs, including colitis, pneumonitis, and endocrinopathy, which can require high-dose steroids or multidisciplinary management.58 The need for frequent infusions also imposes time and logistical burdens on patients and care teams.71
With FDA approval in 2022, nadofaragene firadenovec is the first intravesical gene therapy for BCG-unresponsive NMIBC. Specifically, it is indicated for adult patients with CIS with or without concomitant papillary tumors who have failed adequate BCG therapy.60,72
The mechanism of action of nadofaragene firadenovec involves the use of a nonreplicating adenoviral vector to deliver the human IFN-α2b gene directly to the urothelium (Figure 2).73 This leads to the local production of IFN and stimulation of both innate and adaptive anti-tumor immune responses, including recruitment of cytotoxic T cells and the release of key cytokines such as IL-2 and IFN-γ.73,74
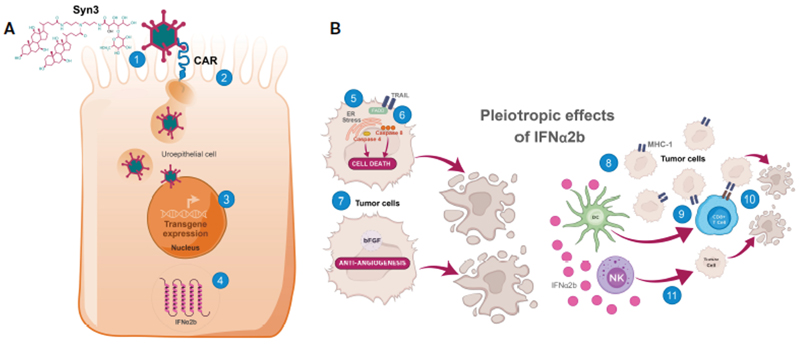 |
| Figure 2. Mechanism of action for nadofaragene firadenovec.73
A. Internalization of adenoviral vector into uroepithelial cells and transcription/translation of IFNa2b. (1) Syn3 promotes adenoviral vector access to uroepithelial cells; (2) After CAR-mediated endocytosis into the uroepithelial cell and escape from endosomes, the adenoviral capsid translocates to the nuclear envelope where it disassembles, and the human IFNa2b transgene is imported into the nucleus; (3) After import into the nucleus, IFNa2b cDNA is transcribed into mRNA; (4) IFNa2b mRNA is translated, leading to sustained production of IFNa2b.
B. IFNa2b exerts pleiotropic antitumor effects directly and indirectly in the tumor microenvironment. Direct cytotoxic effects include (5) induced ER stress in bladder cancer cells, leading to caspase 4 activation and cell death, (6) increased expression of TRAIL, leading to caspase 8 activation and cell death, and (7) antiangiogenic effects through downregulation of growth factors including bFGF, leading to tumor hypoxia and central necrosis. Direct immunomodulatory effects are elicited through (8) upregulation of the presentation of surface tumor-associated antigens via augmentation of MHC-I class molecules, increasing the immunogenicity of tumor cells. Indirect immunomodulatory effects occur through (9) stimulation of Dcpriming of cytotoxic CD8+ T cells, which (10) kill MHC-I+ tumors, and (11) increased antitumor activity of NK cells, which preferentially kill BCa cells lacking MHC-I (downregulation or loss of MHC-I is common in BCa cells).
BCa, bladder cancer; bFGF, basic fibroblast growth factor; CAR, coxsackievirus and adenovirus receptor; CD, cluster of differentiation; cDNA, complementary deoxyribonucleic acid; DC, dendritic cell; ER, endoplasmic reticulum; FADD, Fas-associated protein with death domain; IFNa2b, interferon a2b; MHC-I, major histocompatibility complex class I; mRNA, messenger ribonucleic acid; NK, natural killer; TRAIL, tumor necrosis factorâ??related apoptosis-inducing ligand.
Reused with permission. Creative Commons 4.0. https://creativecommons.org/licenses/by/4.0/
|
Nadofaragene firadenovec is administered as a 75-mL intravesical instillation every 3 months in the urologist’s office.60
In the pivotal phase 3 trial (NCT02773849), which enrolled 157 heavily pretreated patients (50% with ≥3 prior BCG courses), 53.4% of the CIS cohort achieved a CR within 12 months of their first dose, with all responses observed by month 3. Among responders, 45.5% maintained response at 12 months.61 The median DoR was 9.7 months, and the 24-month recurrence-free survival (RFS) rate was approximately 17%. For those with high-grade Ta or T1 tumors, 72.9% were high-grade recurrence-free at month 3 and 60% at 12 months, with a median high-grade RFS of 12.35 months. Notably, 75% of patients were cystectomy-free at 12 months.61
The safety profile for nadofaragene firadenovec has been favorable: most AEs were low-grade (catheter site discharge, fatigue, bladder spasms, urgency), grade 3 events were rare, and no grade 4 or 5 treatment-related events were observed.60,61 Five-year follow-up confirmed an RFS rate of 13% at 57 months in the CIS cohort and 33% in the Ta/T1 cohort, alongside 49% cystectomy-free survival at 60 months and no severe AEs.62
Early real-world data support the pivotal trial. For example, a 2025 Mayo Clinic study demonstrated CR rates of 79% at 3 months and 84% at 7.3 months, with safety consistent with earlier reports.75 The ABLE-41 phase 4 observational study is underway and will report on real-world use, treatment patterns, and long-term outcomes when completed in December 2025.76
Practical considerations with nadofaragene firadenovec involve cold-chain shipping and gene therapy-specific handling protocols. This agent should not be administered in cases of gross hematuria, catheter trauma, or within 14 days of bladder surgery, to minimize risk for systemic exposure.60
Based on phase 3 efficacy data (CR ≥50%) and favorable long-term safety, nadofaragene firadenovec has been included in updated guidance from the NCCN, AUA, and the Society of Urologic Oncology, which together support its role as the preferred gene therapy for eligible BCG-unresponsive NMIBC patients.16,27,77
Nogapendekin alfa inbakicept is a recombinant IL-15 receptor immunotherapy approved by the FDA in 2024 for use in combination with BCG for patients with BCG-unresponsive NMIBC with CIS, with or without papillary tumors.63 It is a recombinant fusion protein engineered as an IL-15 receptor superagonist, which works synergistically with BCG to drive immune-mediated tumor clearance. Specifically, nogapendekin alfa inbakicept binds to and signals through the IL2Rβ/γ receptor complex on immune effector cells—including natural killer cells and CD8+ cytotoxic T lymphocytes—resulting in sustained cytotoxic activity within the bladder wall. The end result is amplification of antitumor responses originally triggered by BCG, enhancing cytokine release and tumor infiltration by immune effector cells (Figure 3).64,78
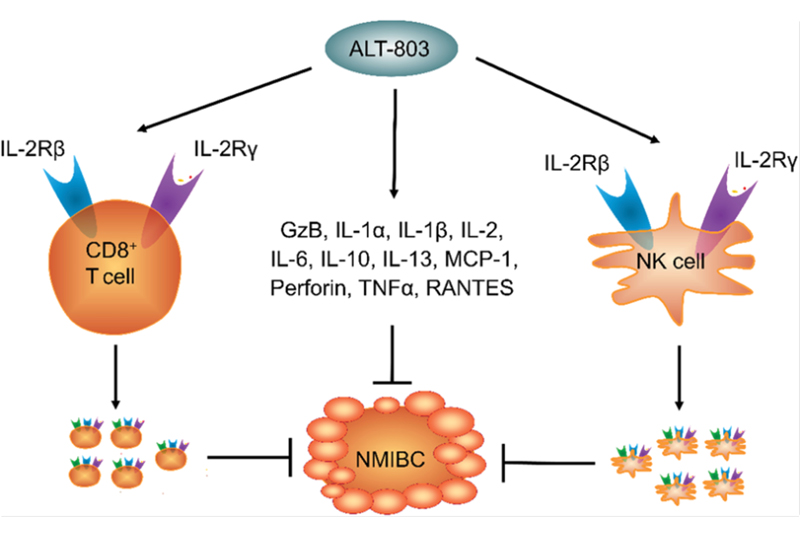 |
| Figure 3. Mechanism of action for nogapendekin alfa inbakicept.64,78 ALT-803, nogapendekin alfa inbakicept; GzB, granzyme B; IL, interleukin; MCP, monocyte chemoattractant protein; NMIBC, non–muscle-invasive bladder cancer; NK, natural killer; RANTES, regulated upon activation, normal T cell expressed and secreted; TNF, tumor necrosis factor. Reused with permission. Creative Commons 4.0. https://creativecommons.org/licenses/by/4.0/ |
Nogapendekin alfa inbakicept is administered via intravesical instillation as 6 weekly induction doses (given with standard BCG instillations) followed by monthly maintenance for up to 12 months in patients who derive benefit or until recurrence or progression.63
Efficacy and safety were reported in the QUILT 3.032 phase 2/3 open-label study, for which the main efficacy population included 82 patients with CIS (with or without papillary tumors) and 77 patients with high-grade papillary (Ta/T1) NMIBC.64 CR was seen in 71% of patients with CIS, with a median DoR of 26.6 months, and 45% of initial responders remaining disease-free at 12 months. In the high-grade papillary group, the median disease-free survival was 19.3 months. The most common AEs were urinary in nature or immune-related and were generally manageable and consistent with the effects of other intravesical immunotherapies.
Treatment-emergent adverse events (TEAEs) were mostly lower urinary tract symptoms, including dysuria, pollakiuria (frequent urination), and hematuria.64 Grade 3 TEAEs included 4 cases of hematuria, 3 of UTI, 3 immune-related events, and 1 each of dysuria and pollakiuria. One grade 5 event (fatal cardiac arrest) was attributed to underlying comorbidity rather than drug toxicity, according to investigators. The final readout of the phase 3 QUILT 3.032 study is anticipated in March 2029.
There are several practical considerations for nogapendekin alfa inbakicept/BCG therapy. Due to its mechanism and the need for synergy, nogapendekin alfa inbakicept is not approved as monotherapy and should not be used without concurrent BCG; consequently, therapy is limited by BCG availability and ongoing international supply constraints. Although generally well tolerated, IL-15 superagonists can produce systemic immune symptoms, such as fever and fatigue, at higher rates than BCG alone and may require supportive management. As a biologic, nogapendekin alfa inbakicept must be ordered through a specialty pharmacy, and initial treatment coordination can be affected by pharmacy logistics and delivery timelines.63,64
The most recent approval, TAR-200, is an intravesical delivery system that uses osmotic minitablets to provide continuous local release of gemcitabine over several weeks, at a dose of 225 mg.65
Approval was based on the phase 2b SunRISe-1 trial, which enrolled patients with BCG-unresponsive high-risk NMIBC with CIS with or without papillary disease. In the monotherapy cohort (n=85), the CR rate was 82.4%, with 45.9% of patients maintaining response at 12 months, and the median DoR was 25.8 months.66 AEs were mostly low-grade and included pollakiuria, dysuria, micturition urgency, and UTI.
The gemcitabine intravesical system is inserted once every 3 weeks for up to 6 months (8 doses), followed by once every 12 weeks for up to 18 months (6 doses), or until the development of persistent or recurrent high-grade NMIBC, disease progression, or unacceptable toxicity.65 Contraindications include bladder perforation and prior hypersensitivity reaction to gemcitabine. 7
The combination of intravesical gemcitabine (a nucleoside analog that inhibits DNA synthesis) and docetaxel (a microtubule stabilizer preventing mitosis) is not FDA-approved for use in NMIBC but is widely used off-label for patients with BCG-unresponsive or recurrent intermediate/high-risk disease, especially if gene therapies or immunotherapy are unavailable. This sequential regimen is listed in both AUA and NCCN guidelines.16,27
The clinical evidence base for this use has evolved over 2 decades. Initial single-center data in heavily pretreated, BCG-unresponsive NMIBC showed high-grade RFS of 34% at 2 years,79 and subsequent multi-institutional studies confirmed 2-year high-grade RFS rates around 46%.67 Long-term follow-up of these cohorts demonstrated a 5-year bladder preservation rate of 75% and a cancer-specific survival rate of 91%.80 More recent retrospective and comparative studies, including multicenter studies, have shown 3-month CR rates as high as 92% for treatment-naïve patients, multi-institutional 2-year RFS rates between 46% and 54%, and 5-year bladder preservation rates consistently exceeding 70%.69,70,80 A recent international comparative analysis found that sequential gemcitabine-docetaxel provided significantly higher rates of progression-free, cystectomy-free, and cancer-specific survival than additional BCG in BCG-unresponsive disease, with similar overall survival and favorable tolerability.70
This drug combination is being studied in the BRIDGE trial, the first randomized phase 3 study directly comparing intravesical gemcitabine + docetaxel with intravesical BCG in BCG-naive high-grade NMIBC.81 This is the first large trial to test for noninferiority to BCG in event-free survival (EFS) in the frontline setting.
For most patients, intravesical gemcitabine/docetaxel is administered via weekly induction instillations for 6 weeks, with monthly maintenance as appropriate. Side effects are typically mild and localized, such as dysuria or urinary frequency, and the regimen is well tolerated even in elderly or comorbid populations.67,70,79
Multiple novel agents and platforms are now in advanced clinical development for patients with NMIBC—particularly those who are unresponsive to or ineligible for BCG (Figure 4). These new therapies focus on enhancing response durability, minimizing recurrence risk, and providing alternatives to surgery.
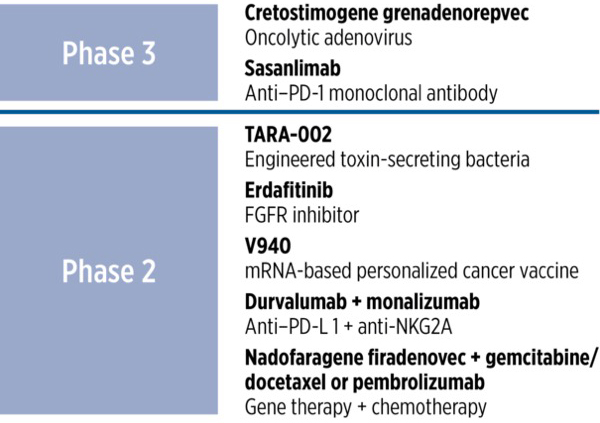 |
| Figure 4. Emerging therapies for non–muscle-invasive bladder cancer.
FGFR, fibroblast growth factor receptor; mRNA, messenger RNA; NK, natural killer; PD, programmed death; PD-L, programmed death ligand.
|
- Cretostimogene grenadenorepvec (CG0070) is a tumor-selective oncolytic adenovirus engineered to replicate in cells with defective retinoblastoma pathway function and locally express granulocyte-macrophage colony-stimulating factor to amplify anti-tumor immunity. In the pivotal phase 3 BOND-003 trial (N=110, high-risk, BCG-unresponsive NMIBC), an interim CR rate of 75.7% was reported, with high durability at 3 and 6 months and a favorable tolerability profile.82 Updated AUA 2024 data confirmed a 75.2% CR, 96.7% 12-month progression-free survival, and no severe TRAEs.83 Ongoing studies are evaluating CG0070 in intermediate-risk disease (PIVOT-006) and in combination with pembrolizumab (CORE-001).
- Sasanlimab is a subcutaneously administered anti-PD-1 monoclonal antibody. In the phase 3 CREST trial (N=1055; high-risk, BCG-naïve NMIBC), sasanlimab plus SOC BCG resulted in a meaningful improvement in EFS, with a hazard ratio of 0.68 and a 36-month EFS of 82.1% vs 74.8% with BCG alone. Benefits were consistent across CIS, Ta, and T1 subgroups and regardless of PD-L1 status. Safety findings were consistent with known BCG and checkpoint inhibitor profiles.84,85
- TARA-002 is a purified, inactivated strain of Streptococcus pyogenes that elicits a local immune response. Interim analysis of the phase 2 ADVANCED-2 study showed encouraging activity in patients with CIS who were BCG-unresponsive or -naïve.86
- Erdafitinib is an oral pan-fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitor, already approved in metastatic urothelial carcinoma, now in evaluation for FGFR-mutated NMIBC. A phase 2 trial is comparing erdafitinib with intravesical chemotherapy in patients who have FGFR-altered high-risk NMIBC (NCT04172675). Erdafitinib is also the active agent used in TAR-210, a novel drug-releasing intravesical delivery system designed to provide sustained local release directly into the bladder over a 3-month period. This therapy showed high CR rates and safety in FGFR3-altered intermediate-risk tumors in early study.87 The Moonrise-1 phase 3 study is now evaluating device against standard intravesical therapies for patients with FGFR-altered intermediate-risk NMIBC, and results will further clarify its role in clinical practice.88
- V940 (mRNA neoantigen vaccine) is a personalized mRNA vaccine encoding patient-specific tumor neoantigens, designed to stimulate anti-tumor T-cell responses. A phase 2 study is evaluating V940 co-administered with BCG in high-risk NMIBC (NCT06833073).
- Durvalumab + monalizumab combines anti-PD-L1 (durvalumab) plus anti-NKG2A (monalizumab) mechanisms and is intended to enhance both adaptive and innate anti-tumor immunity. The phase 2 ENHANCE study is assessing efficacy in BCG-exposed CIS or papillary NMIBC (NCT02671435).
- Nadofaragene firadenovec + gemcitabine/docetaxel or pembrolizumab represents another multimodal regimen seeking to improve outcomes beyond current monotherapy approaches. The ABLE-22 randomized phase 2 trial is examining this combination in high-grade, BCG-unresponsive NMIBC (NCT06545955). The protocol allows re-induction for patients without CR at 3 months, assessing for delayed response at 6 months. The design was presented at the ASCO GU Cancers Symposium, but as of mid-2025, no efficacy or safety data have been released; topline results are expected in July 2028.89
Effective management of NMIBC demands more than technical proficiency; it requires that clinicians facilitate patient understanding, empower shared decision-making, and actively address health system barriers. These elements become especially urgent given the complexity and emotional toll of NMIBC treatment decisions. Most patients are elderly, medically complex, and face difficult choices between emerging bladder-sparing therapies and radical cystectomy. The burden of lifelong surveillance, unfamiliarity with cancer risk, and misunderstandings about trade-offs among therapies heighten anxiety and threaten adherence to follow-up. Emotional stress, including fear of recurrence and “scanxiety,” can adversely affect QoL and even outcomes, underscoring the need for empathetic, proactive communication at every stage of care.2,14,90
Patient-centered communication begins by soliciting patients’ values, concerns, and goals. Clinicians should strive to explain diagnosis, prognosis, and therapeutic choices in plain language, adapting explanation to the individual’s background and health literacy. Rather than relying exclusively on technical language, physicians are encouraged to use conversational approaches—asking patients to describe in their own words what they understand about their disease and treatment options, and correcting misunderstandings as needed. It is especially important to normalize the emotional effects of an NMIBC diagnosis, discuss anticipated follow-up requirements, and offer referrals for psychosocial support when distress is reported.90
When presenting treatment options, including gene therapies, immunotherapy, or surgery, clinicians should neutrally describe the anticipated benefits, risks, frequency of administration, and monitoring needs for each approach. Discussing logistics and safety monitoring for novel agents can demystify treatment and foster adherence. Where appropriate, evidence-based decision aids and visual summaries can be provided to help patients weigh choices. Validated tools (eg, risk calculators or institutional treatment algorithm charts) facilitate clearer discussion of recurrence and progression risk in the context of patient values. Involving family or caregivers and inviting questions further strengthens patient engagement.47,91,92
Health system navigation is an essential complement to effective communication and shared decision-making. New therapies, such as nadofaragene firadenovec (J-code: J9355) or nogapendekin alfa inbakicept (J-code: J9028), may require specialist pharmacy handling, prior authorizations, and coordination among multiple departments. Anticipating insurance or formulary barriers, financial counseling, and assistance from dedicated nurse navigators or administrative staff can minimize delays and patient frustration. Institutional protocols for biosafety, product storage, and staff training must be followed for gene therapies, with clear explanation to patients about scheduling and peri-procedural requirements.
For veterans or patients navigating large health systems, clinicians should proactively clarify local access pathways and help patients connect with resources unique to their care setting. Patients in the Veterans Health Administration and similar systems may benefit from early engagement with patient advocates, social workers, or resource navigators who can facilitate access to care, resolve logistical hurdles, and provide tailored information about benefits and entitlements. The efficacy of patient navigation programs in improving cancer treatment adherence, outcomes, and patient satisfaction is well-supported by systematic reviews.93 Engagement with peer support groups—either veteran-specific or general cancer support networks—has also been shown to improve coping skills and satisfaction with care.94,95
Because financial stress and out-of-pocket costs can lead patients to delay or forego necessary cancer care, open, nonjudgmental dialogue around drug costs, insurance coverage, travel expenses, and other financial concerns is crucial. This risk, commonly termed “financial toxicity,” has been widely recognized as a critical component of modern cancer care. Proactive communication and referral to financial counselors or navigators can help patients anticipate, mitigate, and manage these burdens, which may be particularly significant for those on fixed incomes, facing multiple comorbidities, or transitioning from military to civilian insurance coverage.94,96,97
A structured approach to these challenges might include:
- Conducting scheduled, uninterrupted counseling sessions dedicated to decision-making
- Summarizing follow-up and treatment pathways in both written and visual forms
- Documenting the use of shared decision-making aids and patient preferences in the medical record
- Coordinating with multidisciplinary teams (including nursing, pharmacy, navigation support, and advocacy organizations) throughout the care continuum
- Referring patients to key resources such as Cancer Care’s dedicated veteran guide95 and NCI’s financial navigation resources, which offer practical support for addressing access and affordability issues96
By intentionally centering the patient in all aspects of NMIBC management and anticipating both personal and health system barriers, clinicians can optimize outcomes—ensuring equitable access to innovative therapies, improving adherence, and maximizing quality of life throughout the cancer journey.
|
 He presents to the VA primary care clinic reporting intermittent painless hematuria. Urinalysis and urine cytology are abnormal. Referral to urology and subsequent cystoscopy reveal multiple papillary lesions and CIS. John undergoes complete TURBT. Staging confirms high-risk NMIBC CIS with associated high-grade Ta lesions, no invasion into the lamina propria, and negative upper-tract imaging. He starts standard induction BCG therapy (6 weekly instillations) and a 3-month maintenance course. However, at his 6-month surveillance cystoscopy, persistent CIS and positive cytology are observed. He is now classified as having BCG-unresponsive NMIBC. Radical cystectomy is discussed as a guideline-recommended option; however, John declines surgery due to concerns about QoL and PTSD-related anxiety. Instead, after a multidisciplinary discussion including the urology team, oncology nurse navigator, and mental health support, John opts for bladder-sparing therapy with nadofaragene firadenovec, administered as 75-mL intravesical instillations every 3 months. At 3 and 6 months post-treatment, John is recurrence-free with negative cystoscopy, cytology, and bladder biopsies. Mild urinary urgency is observed, but no serious AEs occur. By 12 months, John is still cancer-free, has resumed part-time volunteering at a military museum, and reports improved emotional well-being. He credits the availability of an effective nonsurgical option with maintaining his physical health and managing his PTSD. Key Points
|
NMIBC remains a burdensome malignancy for patients, clinicians, and the health care system due to its high rates of recurrence, progression, and the psychosocial consequences of both treatment and surveillance. Although BCG has served as the backbone of therapy for decades, the persistent challenge of BCG-unresponsive disease has driven innovation in the field. The emergence of intravesical gene therapies and novel immunomodulatory regimens now offers patients meaningful, bladder-sparing options that are supported by clinical trial data and growing real-world experience. Individualized risk stratification grounded in current staging and grading paradigms guides the use of such therapies and ensures that patients receive optimal, evidence-based care.
Success in NMIBC management now requires not only mastery of evolving treatment pathways and multidisciplinary decision-making, but also the intentional application of patient-centered communication, shared decision-making, and effective health system navigation. By staying updated on therapeutic advancements and proactively addressing patient values, logistical barriers, and disparities in access, clinicians can help maximize cure rates, QoL, and long-term outcomes for all individuals confronting NMIBC.
- American Cancer Society. Cancer facts & figures 2025. Accessed September 3, 2025. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2025/2025-cancer-facts-and-figures-acs.pdf
- National Cancer Institute. SEER cancer statistics review, 1975–2021. Surveillance, Epidemiology, and End Results Program. Accessed April 4, 2025. https://seer.cancer.gov/archive/csr/1975_2018/index.html
- National Cancer Institute. PDQ® Bladder cancer treatment – health professional version. Accessed April 6, 2025. www.cancer.gov/types/bladder/hp/bladder-treatment-pdq
- ACS Cancer Prevalence. https://www.cancer.org/cancer/survivorship/cancer-prevalence.html
- Yee DS, Ishill NM, Lowrance WT, Herr HW, Elkin EB. Ethnic differences in bladder cancer survival. Urology. 2011;78(3):544-549.
- Majumder A, Erim D, Kaur A, Orr LD. Geographic and racial disparities in bladder cancer in the U.S. Presented at: American Association for Cancer Research; April 25-28, 2025; Chicago, IL. Poster 3621.
- National Cancer Institute. SEER training modules. Updated April 22, 2025. Accessed July 15, 2025. https://training.seer.cancer.gov/bladder/
- Grabe-Heyne K, Henne C, Mariappan P, Geiges G, Pöhlmann J, Pollock RF. Intermediate and high-risk non-muscle-invasive bladder cancer: an overview of epidemiology, burden, and unmet needs. Front Oncol. 2023;13:1170124.
- Teoh JY, Kamat AM, Black PC, Grivas P, Shariat SF, Babjuk M. Recurrence mechanisms of non-muscle-invasive bladder cancer - a clinical perspective. Nat Rev Urol. 2022;19(5):280-294.
- Williams SB, Howard LE, Foster ML, et al. Estimated costs and long-term outcomes of patients with high-risk non–muscle-invasive bladder cancer treated with Bacillus Calmette-Guérin in the Veterans Affairs health system. JAMA Netw Open. 2021;4(3):e213800.
- VA News. Bladder cancer and veterans: what you need to know. May 11, 2022. Accessed April 7, 2025. https://news.va.gov/103200/bladder-cancer-and-veterans-what-you-need-to-know/
- Williams SB, Janes JL, Howard LE, et al. Exposure to agent orange and risk of bladder cancer among US veterans. JAMA Netw Open. 2023;6(6):e2320593.
- Kronstedt S, Cathey J, Chiu CB, et al. Exposures and bladder cancer risk among military veterans: a systematic review and meta-analysis. Urology. 2024;194:270-277.
- Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet. 2016;388(10061):2796-2810.
- Babjuk M, Burger M, Comperat EM, et al. European Association of Urology guidelines on non–muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ) – 2022 update. Eur Urol. 2022;81(1):75-94.
- National Comprehensive Cancer Network. Bladder Cancer Guidelines, Version 2.2024.
- Cahill EM, Chua K, Doppalapudi SK, Ghodoussipour S. The use of blue-light cystoscopy in the detection and surveillance of nonmuscle invasive bladder cancer. Curr Urol. 2022;16(3):121-126.
- Guldhammer CS, Vásquez JL, Kristensen VM, et al. Cystoscopy accuracy in detecting bladder tumors: a prospective video-confirmed study. Cancers (Basel). 2023;16(1):160.
- Lotan Y, Roehrborn CG. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: results of a comprehensive literature review and meta-analyses. Urology. 2003;61(1):109-118.
- Dimashkieh H, Wolff DJ, Smith TM, Houser PM, Nietert PJ, Yang J. Evaluation of Urovysion and cytology for bladder cancer detection: a study of 1835 paired urine samples with clinical and histologic correlation. Cancer Cytopathol. 2013;121(10):591-597.
- Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA. 2005;293(7):810-816.
- Hu X, Li G, Wu S. Advances in diagnosis and therapy for bladder cancer. Cancers (Basel). 2022;14(13):3181.
- American Cancer Society. Can bladder cancer be found early? Revised March 12, 2024. Accessed April 7, 2025. www.cancer.org/cancer/types/bladder-cancer/detection-diagnosis-staging/detection.html
- Das S, Gu L, Eve CT, et al. The impact of blue light cystoscopy use among nonmuscle invasive bladder cancer patients in an equal access setting: implications on recurrence and time to recurrence. Clin Genitourin Cancer. 2023;21(6):711.e1-711.e6.
- Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466-465.
- Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs-Part B: prostate and bladder tumours. Eur Urol. 2016;70(1):106-119.
- Holzbeierlein J, Bixler BR, Buckley DI, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline: 2024 amendment. J Urol. 2024;211(4):533-538.
- Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol. 2004;171(6 pt 1):2186-90.
- Sylvester R. A single immediate instillation of chemotherapy for non-muscle invasive bladder cancer: in all patients? Transl Androl Urol. 2018;7(suppl 1):S138-S140.
- Prasad SM, Shishkov D, Mihaylov NV, et al. Primary chemoablation of recurrent low-grade intermediate-risk nonmuscle-invasive bladder cancer with UGN-102: a single-arm, open-label, phase 3 trial (ENVISION). J Urol. 2025;213(2):205-216.
- Urogen Pharma. UroGen announces results from subgroup analyses of the pivotal ENVISION trial evaluating impact of baseline tumor burden and focality on response to UGN-102 [press release]. February 14, 2025. Accessed July 15, 2025. https://investors.urogen.com/news-releases/news-release-d etails/urogen-announces-results-subgroup-analyses-pivotal-envision
- Zusduri (mitomycin) prescribing information. UroGen Pharma. June 2025.
- Prasad SM, Huang WC, Shore ND, et al. Treatment of Low-grade intermediate-risk nonmuscle-invasive bladder cancer with UGN-102 ± transurethral resection of bladder tumor compared to transurethral resection of bladder tumor monotherapy: a randomized, controlled, phase 3 trial (ATLAS). J Urol. 2023;210(4):619-629.
- Tice BCG (BCG live for intravesical use) prescribing information. Rahway, NJ: Merck & Co., Inc.; Aug 2025.
- Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
- Sylvester RJ, van der Meijden AP, Witjes JA, Kurth K. Bacillus Calmette-Guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol. 2005;174(1):86-91.
- Lahoud H, Okullo A, Rutherford C, et al. Symptoms and side effects of Bacille Calmette–Guerin therapy for non-muscle invasive bladder cancer as reported by patients: a systematic review. Cancers (Basel). 2025;17(2):160.
- Waked R, Choucair J, Chehata N, Haddad E, Saliba G. Intravesical Bacillus Calmette-Guérin (BCG) treatment’s severe complications: a single institution review of incidence, presentation and treatment outcome. J Clin Tuberc Other Mycobact Dis. 2020;19:100149.
- Cleveland Clinic. Bacillus Calmette-Guerin (BCG) treatment. Reviewed March 21, 2025. Accessed August 4, 2025. https://my.clevelandclinic.org/health/treatments/17908-bacillus-calmette-guerin-bcg-treatment
- American Urological Association. BCG shortage info. Accessed July 15, 2025. www.auanet.org/about-us/bcg-shortage-info
- Klassen Z. ASCO GU 2024: BCG Shortage: What’s on the horizon to replace? Presented at: American Society of Clinical Oncology Genitourinary Cancers Symposium; January 25-27, 2024; San Francisco, CA.
- Hollasch M. Bladder cancer care shifts as BCG shortage lingers, with investigative combinations and tools at the forefront. OncLive. February 10, 2025. Accessed June 25, 2025. www.onclive.com/view/bladder-cancer-care-shifts-as-bcg-shortage-lingers-with-investigative-combinations-and-tools-at-the-forefront
- Gupta A, Verma S, Gupta S. Treatment strategies for BCG-unresponsive non-muscle invasive bladder cancer. Ann Urol Oncol. 2024;7(3):97-109.
- Hwang TJ, Davies BJ, Preston MA. Advancing patient-centered outcomes and equity in clinical trials for BCG-unresponsive nonmuscle invasive bladder cancer. JAMA Oncol. 2023;9(11):1491-1492.
- US Department of Health and Human Services. BCG-unresponsive nonmuscle invasive bladder cancer: developing drug and biological products for treatment - guidance for industry. August 2024. Accessed August 4, 2025. www.fda.gov/media/101468/download
- Li R, Hensley PJ, Gupta S, et al. Bladder-sparing therapy for Bacillus Calmette-Guérin-unresponsive non-muscle-invasive bladder cancer: International Bladder Cancer Group recommendations for optimal sequencing and patient selection. Eur Urol. 2024;86(6):516-527.
- Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55(1):164-174.
- Hautmann RE, Hautmann SH, Hautmann O. Complications associated with urinary diversion. Nat Rev Urol. 2011;8(12):667-677.
- Maibom SL, Joensen UN, Poulsen AM, Kehlet H, Brasso K, Røder MA. Short-term morbidity and mortality following radical cystectomy: a systematic review. BMJ Open. 2021;11(4):e043266.
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666-675.
- Lenis AT, Lec PM, Chamie K, MSHS MD. Bladder cancer: a review. JAMA. 2020;324(19):1980-1991.
- Tyson MD 2nd, Barocas DA. Quality of life after radical cystectomy. Urol Clin North Am. 2018;45(2):249-256.
- Bahlburg H, Reicherz A, Reike M, et al. A prospective evaluation of quality of life, psychosocial distress, and functional outcomes two years after radical cystectomy and urinary diversion in 842 German bladder cancer patients. J Cancer Surviv. 2025;19(3):1102-1110.
- Modh RA, Mulhall JP, Gilbert SM. Sexual dysfunction after cystectomy and urinary diversion. Nat Rev Urol. 2014;11(8):445-453.
- Pederzoli F, Campbell JD, Matsui H, Sopko NA, Bivalacqua TJ. Surgical factors associated with male and female sexual dysfunction after radical cystectomy: what do we know and how can we improve outcomes? Sex Med Rev. 2018;6(3):469-481.
- Bahlburg H, Hellmann T, Tully K, et al. Psychosocial distress and quality of life in patients after radical cystectomy - one year follow-up in 842 German patients. J Cancer Surviv. 2024;18(5):1600-1607.
- Gore JL, Wolff EM, Nash MG, et al. Radical cystectomy versus bladder-sparing therapy for recurrent high-grade non-muscle invasive bladder cancer: results from the comparison of intravesical therapy and surgery as treatment options (CISTO) study. J Urol. 2025;213(5S2):e3-e4.
- Keytruda (pembrolizumab) prescribing information. Whitehouse Station, NJ: Merck & Co, Inc. Jan 2020.
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021;22(7):919-930.
- Adstiladrin (nadofaragene firadenovec) prescribing information. Kastrup, Denmark: Ferring Pharmaceuticals. Aug 2024.
- Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021;22(1):107-117.
- Narayan VM, Boorjian SA, Alemozaffar M, et al. Efficacy of intravesical nadofaragene firadenovec for patients with Bacillus Calmette-Guérin-unresponsive nonmuscle-invasive bladder cancer: 5-year follow-up from a phase 3 trial. J Urol. 2024;212(1):74-86.
- Anktiva (nogapendekin alfa inbakicept-pmln) prescribing information. Culver City, CA: ImmunityBio, Inc. Apr 2024.
- Chamie K, Chang SS, Kramolowsky E, et al. IL-15 superagonist NAI in BCG-unresponsive non-muscle-invasive bladder cancer. NEJM Evid. 2023;2(1):EVIDoa2200167.
- Inlexzo (gemcitabine intravesical system) prescribing information. Horsham, PA: Janssen Biotech, Inc. Sept 2025.
- Daneshmand S, Van der Heijden MS, Jacob JM, et al. TAR-200 for Bacillus Calmette-Guérin-unresponsive high-risk non-muscle-invasive bladder cancer: results from the phase IIb SunRISe-1 study. J Clin Oncol. 2025:JCO2501651.
- Steinberg RL, Thomas LJ, Brooks N, et al. Multi-institution evaluation of sequential gemcitabine and docetaxel as rescue therapy for nonmuscle invasive bladder cancer. J Urol. 2020;203(5):902-909.
- Steinberg RL, Packiam VT, Thomas LJ, et al. Intravesical sequential gemcitabine and docetaxel versus Bacillus Calmette-Guerin (BCG) plus interferon in patients with recurrent non-muscle invasive bladder cancer following a single induction course of BCG. Urol Oncol. 2022;40(1):9.e1-9.e7.
- McElree IM, Steinberg RL, Mott SL, O’Donnell MA, Packiam VT. Comparison of sequential intravesical gemcitabine and docetaxel vs Bacillus Calmette-Guérin for the treatment of patients with high-risk non-muscle-invasive bladder cancer. JAMA Netw Open. 2023;6(2):e230849.
- Taylor J, Kamat AM, Annapureddy D, et al. Oncologic outcomes of sequential intravesical gemcitabine and docetaxel compared with Bacillus Calmette-Guérin in patients with Bacillus Calmette-Guérin-unresponsive non-muscle invasive bladder cancer. Eur Urol Oncol. 2025;8(2):469-476.
- Aguiar-Ibáñez R, Scherrer E, Grebennik D, et al. Time and productivity loss associated with immunotherapy infusions for the treatment of melanoma in the United States: a survey of health care professionals and patients. BMC Health Serv Res. 2023;23(1):136.
- US Food and Drug Administration. FDA approves first adenoviral vector-based gene therapy for high-risk Bacillus Calmette-Guerin unresponsive non-muscle invasive bladder cancer. Last reviewed December 19, 2022. Accessed September 3, 2025. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-first-adenoviral-vector-based-gene-therapy-high-risk-bacillus-calmette-guerin
- Narayan VM, Meeks JJ, Jakobsen JS, Shore ND, Sant GR, Konety BR. Mechanism of action of nadofaragene firadenovec-vncg. Front Oncol. 2024;14:1359725.
- Lee A. Nadofaragene firadenovec: first approval. Drugs. 2023;83:353-357.
- Moyer J, Durant A, Nguyen M, et al. Real-world outcomes of nadofaragene firadenovec in BCG-unresponsive non-muscle invasive bladder cancer. J Clin Oncol. 2025;43(suppl 5):716.
- Ferring Pharmaceuticals. Phase 4 study evaluating use of Adstiladrin in real-world setting (ABLE-41) [press release]. January 24, 2024. Accessed April 4, 2025. https://ferringusa.com/?press=phase-4-study-evaluating-use-of-adstiladrin-nadofaragene-firadenovec-vncg-in-real-world-setting
- Gontero P, Birtle A, Comperat E, et al. EAU guidelines on non-muscle-invasive bladder cancer (TaT1 and CIS). Accessed March 31, 2025. https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Non-muscle-invasive-Bladder-Cancer-2025.pdf
- Chen W, Liu N, Yuan Y, et al. ALT-803 in the treatment of non-muscle-invasive bladder cancer: preclinical and clinical evidence and translational potential. Front Immunol. 2022;13:1040669.
- Steinberg RL, Thomas LJ, O’Donnell MA, Nepple KG. Sequential intravesical gemcitabine and docetaxel for the salvage treatment of non-muscle invasive bladder cancer. Bladder Cancer. 2015;1(1):65-72.
- Chevuru PT, McElree IM, Mott SL, Steinberg RL, O’Donnell MA, Packiam VT. Long-term follow-up of sequential intravesical gemcitabine and docetaxel salvage therapy for non-muscle invasive bladder cancer. Urol Oncol. 2023;41(3):148.e1-148.e7.
- Kates M, Chu X, Hahn N, et al. background and update for ECOG-ACRIN EA8212: a randomized phase 3 trial of intravesical bacillus calmette-guérin (BCG) versus intravesical docetaxel and gemcitabine treatment in BCG-naïve high-grade non-muscle-invasive bladder cancer (BRIDGE). Eur Urol Focus. 2023;9(4):561-563.
- Uchio E, Lamm D, Shore N, et al. 426 A phase 3, single-arm study of CG0070 in subjects with non-muscle invasive bladder cancer (NMIBC) unresponsive to Bacillus Calmette-Guerin (BCG). J Immunother Cancer. 2021;9(suppl 2):A456.
- Tyson M, Uchio E, Nam J, et al. Pivotal results from BOND-003: a phase 3, single-arm study of intravesical cretostimogene grenadenorepvec for the treatment of high risk, BCG-unresponsive non-muscle invasive bladder cancer. Presented at: 2024 American Urological Association Meeting; May 3-6, 2024; San Antonio, TX.
- Shore ND, Powles T, Bedke J, et al. Sasanlimab in combination with Bacillus Calmette-Guérin improves event-free survival versus Bacillus Calmette-Guérin as standard of care in high-risk non–muscle-invasive bladder cancer: phase 3 CREST study results. J Urol. 2025;213(5S):e1001.
- Pfizer. Pfizer’s sasanlimab combination significantly improves event-free survival in BCG-naïve, high-risk non-muscle invasive bladder cancer [press release]. April 26, 2025. Accessed September 3, 2025. www.pfizer.com/news/press-release/press-release-detail/pfizers-sasanlimab-combination-significantly-improves-event
- Protara Therapeutics announces positive interim results demonstrating durable responses in the ongoing phase 2 ADVANCED-2 trial of TARA-002 in patients with NMIBC [press release]. April 26, 2025. Accessed September 3, 2025. https://ir.protaratx.com/node/11666/pdf
- Vilaseca A, Jayram G, Raventos C, et al. PD48-02: First safety and efficacy results of the TAR-210 erdafitinib intravesical delivery system in patients with non–muscle-invasive bladder cancer with select FGFR alteration. J Urol. 2024;211(5S):e987.
- Li R, Jayram G, Girvin A, et al. Moonrise-1: phase 3 study of TAR-210, an erdafitinib intravesical targeted releasing system, versus intravesical chemotherapy in patients with FGFR-altered intermediate-risk non–muscle-invasive bladder cancer. Urol Oncol. 2025;43(3 suppl):53
- Daneshmand S, Boorjian SA, Meeks JJ, et al. ABLE-22: Safety and efficacy evaluation of nadofaragene firadenovec alone or in combination with chemotherapy or immunotherapy—a randomized, open-label, phase 2 study. J Clin Oncol. 2025;43 (5 suppl): TPS891.
- Fung C, Pandya C, Guancial E, et al. Impact of bladder cancer on health related quality of life in 1,476 older Americans: a cross-sectional study. J Urol. 2014;192(3):690-695.
- Memorial Sloan Kettering Cancer Center. Targeted therapy for bladder cancer. Accessed July 15, 2025. www.mskcc.org/cancer-care/types/bladder/treatment/targeted-therapy-bladder
- Bladder Cancer Advocacy Network. Downloadable resources. Accessed July 15, 2025. https://bcan.org/downloadable-resources/
- Jojola EC, Cheng H, Wong LJ, Paskett ED, Freund KM, Johnston FM. Efficacy of patient navigation in cancer treatment: a systematic review. JONS. 2017;8(3).
- Sumlin AB, Houjaij A, Darwish OM, Camacho S, Groman A, Fayazi Z. Financial toxicity and strain among veteran and civilian men with prostate cancer. Arch Clin Biomed Res. 2023;7(3):301-205.
- [No author listed]. Building connections as a veteran: resources and support. Cancer Care. Updated July 12, 2022. Accessed August 5, 2025. www.cancercare.org/publications/340-building_connections_as_a_veteran_resources_and_support
- Phillips C. Financial navigators reduce the cost of cancer care. National Cancer Institute. April 21, 2023. Accessed August 5, 2025. www.cancer.gov/news-events/cancer-currents-blog/2023/cancer-care-financial-navigation-saves-money
- de Souza JA, Yap BJ, Wroblewski K, et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: the validation of the COmprehensive Score for financial Toxicity (COST). Cancer. 2017;123(3):476-484.
false
*You must be logged in to see results data.
- Which of the following is the single most important modifiable risk factor for bladder cancer incidence
in the United States?
- Chronic urinary tract infection
- Tobacco use
- Prior pelvic radiation
- Occupational exposure to solvents
Correct answer: BTobacco use accounts for nearly 50% of cases of bladder cancer in the United States. Carcinogenic chemicals from smoking are filtered and concentrated in the urine, prolonging exposure to the bladder lining and increasing mutational risk - What is the gold standard first-line diagnostic tool for detecting visible lesions in NMIBC?
- Urine cytology
- Computed tomography urography
- Cystoscopy
- Fluorescence in situ hybridization
Correct answer: CCystoscopy is the gold standard first-line diagnostic tool for detecting visible lesions in NMIBC, offering high sensitivity (approximately 85%-90%) and specificity (87%-100%) for visible urothelial lesions. It is routinely performed in the office setting and permits direct visualization, biopsy, and resection when needed. - Blue light cystoscopy differs from standard (white light) cystoscopy by improving detection of which of
the following?
- Renal cell carcinoma
- Flat carcinoma in situ (CIS)
- Prostate cancer
- Low-grade Ta tumors only
Correct answer: BBlue light cystoscopy is an advanced diagnostic technique that uses hexaminolevulinate, a photosensitizing agent, which causes cancerous cells to fluoresce under blue light, making them more readily distinguishable from normal tissue. This technique can significantly increase sensitivity for detection, particularly flat CIS or papillary tumors, and has been shown to improve clinical outcomes by reducing recurrence and progression rates compared with conventional (white light) cystoscopy. - Which of the following is a drawback to the use of Bacillus Calmette–Guérin (BCG) therapy
in NMIBC?
- Global shortages
- High rate of adverse events, including serious events leading to discontinuation
- Risk for failure (non-responsiveness)
- All of the above
Correct answer: DThe use of BCG therapy in NMIBC is associated with all of the drawbacks listed. Global supply issues are widely acknowledged; >70% of patients experience some side effects, including effects leading to discontinuation in ≤10% of users; and ≤40% of patients may not respond to BCG therapy. - Which patient profile best meets consensus criteria for BCG-unresponsive NMIBC?
- Low-grade Ta tumor after 1 dose of BCG
- Cystitis after 1 maintenance dose
- T2 muscle-invasive progression after transurethral resection of bladder tumor only
- High-grade CIS recurrence at 6 months after adequate BCG induction and maintenance
Correct answer: DThis determination is made only after adequate BCG induction and maintenance. - Which of the following describes the mechanism of action (MOA) of nadofaragene firadenovec?
- Intravesical gene delivery of interferon (IFN)-α2b
- Direct intravesical chemotherapy with mitomycin C
- Systemic immune checkpoint inhibition
- Intravesical delivery of interleukin (IL)-15 superagonist
Correct answer: ANadofaragene firadenovec utilizes a nonreplicating adenoviral vector to deliver the IFN-α2b gene directly into the bladder lining, enabling local production of interferon and anti-tumor immune activation through intravesical gene therapy. Options B, C, and D describe mechanisms of different agents. - Which of the following best describes the mechanism of action of nogapendekin alfa inbakicept?
- Direct DNA damage via nucleoside analog
- Recombinant IL-15 receptor superagonist stimulating natural killer (NK) and T cells
- Oncolytic viral replication in tumor cells
- Programmed death ligand-1 immune checkpoint blockade
Correct answer: BNogapendekin alfa inbakicept is a recombinant fusion protein acting as an IL-15 receptor superagonist, designed to stimulate NK and cytotoxic T cells within the bladder wall. This mechanism synergizes with BCG to amplify anti-tumor immunity in NMIBC. Options A, C, and D describe mechanisms of gemcitabine, cretostimogene grenadenorepvec, and pembrolizumab, respectively. - Which of the following communication strategies would best increase adherence and reduce anxiety for a
patient with NMIBC who expresses fear of recurrence and confusion about treatment logistics?
- Provide generic educational pamphlets and schedule follow-up
- Rely on technical terminology during visits and instruct the patient on how to look up terms they do not understand
- Initiate a structured counseling session using conversational assessment, summarize personalized treatment pathways in both written and visual formats, and proactively refer the patient to nurse navigation and psychosocial resources
- Defer in-depth discussion of treatment options to a pharmacist
Correct answer: CThe best approach incorporates structured, personalized counseling using conversational methods to assess the patient’s understanding and concerns, combined with written and visual aids and proactive referrals to navigation and psychosocial support. This strategy is specifically advocated in the monograph for optimizing patient comprehension, reducing anxiety, and supporting adherence, especially for complex therapies and elderly/veteran populations. Options A, B, and D neglect critical participatory and supportive elements established in contemporary patient-centered care models. - A 68-year-old man with CIS and high-grade Ta NMIBC receives BCG but shows persistent CIS after
6 months. He desires bladder preservation and declines cystectomy. Which therapy, supported by phase 3
trial data and FDA approval, is most appropriate?
- Systemic cisplatin
- Nadofaragene firadenovec
- TAR-200
- Sasanlim
Correct answer: BNadofaragene firadenovec is specifically FDA-approved for BCG-unresponsive CIS, with documented efficacy in patients declining cystectomy.
false